Genomic characterization of mycobacteriophage Giles: evidence for phage acquisition of host DNA by illegitimate recombination
- PMID: 18178732
- PMCID: PMC2258872
- DOI: 10.1128/JB.01657-07
Genomic characterization of mycobacteriophage Giles: evidence for phage acquisition of host DNA by illegitimate recombination
Abstract
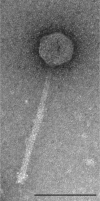
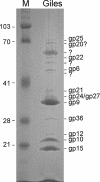
A characteristic feature of bacteriophage genomes is that they are architecturally mosaic, with each individual genome representing a unique assemblage of individual exchangeable modules. Plausible mechanisms for generating mosaicism include homologous recombination at shared boundary sequences of module junctions, illegitimate recombination in a non-sequence-directed process, and site-specific recombination. Analysis of the novel mycobacteriophage Giles genome not only extends our current perspective on bacteriophage genetic diversity, with more than 60% of the genes unrelated to other mycobacteriophages, but offers novel insights into how mosaic genomes are created. In one example, the integration/excision cassette is atypically situated within the structural gene operon and could have moved there either by illegitimate recombination or more plausibly via integrase-mediated site-specific recombination. In a second example, a DNA segment has been recently acquired from the host bacterial chromosome by illegitimate recombination, providing further evidence that phage genomic mosaicism is generated by nontargeted recombination processes.
Figures




References
-
- Angly, F. E., B. Felts, M. Breitbart, P. Salamon, R. A. Edwards, C. Carlson, A. M. Chan, M. Haynes, S. Kelley, H. Liu, J. M. Mahaffy, J. E. Mueller, J. Nulton, R. Olson, R. Parsons, S. Rayhawk, C. A. Suttle, and F. Rohwer. 2006. The marine viromes of four oceanic regions. PLoS Biol. 4e368. - PMC - PubMed
-
- Baranov, P. V., R. F. Gesteland, and J. F. Atkins. 2002. Recoding: translational bifurcations in gene expression. Gene 286187-201. - PubMed
-
- Brussow, H. 2001. Phages of dairy bacteria. Annu. Rev. Microbiol. 55283-303. - PubMed
-
- Campbell, A. 1958. The different kinds of transducing particles in the lambda-gal system. Cold Spring Harbor Symp. Quant. Biol. 2383-84. - PubMed
-
- Casas, V., and F. Rohwer. 2007. Phage metagenomics. Methods Enzymol. 421259-268. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

