Peroxisome proliferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer
- PMID: 18178738
- PMCID: PMC2446782
- DOI: 10.1128/JB.01444-07
Peroxisome proliferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer
Abstract
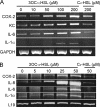
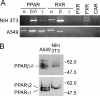
The pathogenic bacterium Pseudomonas aeruginosa utilizes the 3-oxododecanoyl homoserine lactone (3OC(12)-HSL) autoinducer as a signaling molecule to coordinate the expression of virulence genes through quorum sensing. 3OC(12)-HSL also affects responses in host cells, including the upregulation of genes encoding inflammatory cytokines. This proinflammatory response may exacerbate underlying disease during P. aeruginosa infections. The specific mechanism(s) through which 3OC(12)-HSL influences host responses is unclear, and no mammalian receptors for 3OC(12)-HSL have been identified to date. Here, we report that 3OC(12)-HSL increases mRNA levels for a common panel of proinflammatory genes in murine fibroblasts and human lung epithelial cells. To identify putative 3OC(12)-HSL receptors, we examined the expression patterns of a panel of nuclear hormone receptors in these two cell lines and determined that both peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) and PPARgamma were expressed. 3OC(12)-HSL functioned as an agonist of PPARbeta/delta transcriptional activity and an antagonist of PPARgamma transcriptional activity and inhibited the DNA binding ability of PPARgamma. The proinflammatory effect of 3OC(12)-HSL in lung epithelial cells was blocked by the PPARgamma agonist rosiglitazone, suggesting that 3OC(12)-HSL and rosiglitazone are mutually antagonistic negative and positive regulators of PPARgamma activity, respectively. These data identify PPARbeta/delta and PPARgamma as putative mammalian 3OC(12)-HSL receptors and suggest that PPARgamma agonists may be employed as anti-inflammatory therapeutics for P. aeruginosa infections.
Figures
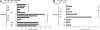
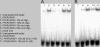


References
-
- Abbas, A. K. 2003. Cellular and molecular immunology, 5th ed. Saunders, Philadelphia, PA.
-
- Adachi, M., R. Kurotani, K. Morimura, Y. Shah, M. Sanford, B. B. Madison, D. L. Gumucio, H. E. Marin, J. M. Peters, H. A. Young, and F. J. Gonzalez. 2006. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 551104-13. - PMC - PubMed
-
- Aranda, A., and A. Pascual. 2001. Nuclear hormone receptors and gene expression. Physiol. Rev. 811269-304. - PubMed
-
- Bals, R., and B. Jany. 2001. Identification of disease genes by expression profiling. Eur. Respir. J. 18882-9. - PubMed
-
- Bastie, C., D. Holst, D. Gaillard, C. Jehl-Pietri, and P. A. Grimaldi. 1999. Expression of peroxisome proliferator-activated receptor PPARδ promotes induction of PPARγ and adipocyte differentiation in 3T3C2 fibroblasts. J. Biol. Chem. 27421920-5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

