CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system
- PMID: 18178850
- PMCID: PMC2185792
- DOI: 10.4049/jimmunol.180.2.1098
CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system
Abstract
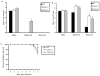
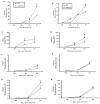
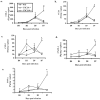
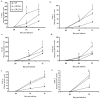
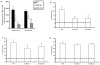
CXCL9 and CXCL10 mediate the recruitment of T lymphocytes and NK cells known to be important in viral surveillance. The relevance of CXCL10 in comparison to CXCL9 in response to genital HSV-2 infection was determined using mice deficient in CXCL9 (CXCL9-/-) and deficient in CXCL10 (CXCL10-/-) along with wild-type (WT) C57BL/6 mice. An increased sensitivity to infection was found in CXCL10-/- mice in comparison to CXCL9-/- or WT mice as determined by detection of HSV-2 in the CNS at day 3 postinfection. However, by day 7 postinfection both CXCL9-/- and CXCL10-/- mice possessed significantly higher viral titers in the CNS in comparison to WT mice consistent with mortality (18-35%) of these mice within the first 7 days after infection. Even though CXCL9-/- and CXCL10-/- mice expressed elevated levels of CCL2, CCL3, CCL5, and CXCL1 in the spinal cord in comparison to WT mice, there was a reduction in NK cell and virus-specific CD8+ T cell mobilization to this tissue, suggesting CXCL9 and CXCL10 are critical for recruitment of these effector cells to the spinal cord following genital HSV-2 infection. Moreover, leukocytes from the spinal cord but not from draining lymph nodes or spleens of infected CXCL9-/- or CXCL10-/- mice displayed reduced CTL activity in comparison to effector cells from WT mice. Thus, the absence of CXCL9 or CXCL10 expression significantly alters the ability of the host to control genital HSV-2 infection through the mobilization of effector cells to sites of infection.
Figures

References
-
- Inagaki K, Daikoku T, Goshima F, Nashiyama Y. Impaired induction of protective immunity by highly virulent herpes simplex type 2 in a murine model of genital herpes. Arch Virol. 2000;145:1989–2002. - PubMed
-
- Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN- -secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–853. - PubMed
-
- Whitley RJ, Miller RL. Immunologic approach to Herpes Simplex Virus. Viral Immunol. 2001;14(2):111–118. - PubMed
-
- Duerst RJ, Morrison LA. Innate immunity to herpes simplex virus type 2. Viral Immunol. 2003;16(4):475–490. - PubMed
-
- MasCasullo V, Fam E, Keller MJ, Herold BC. Role of mucosal immunity preventing genital herpes infection. Viral Immunol. 2005;18(4):595–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

