The ubiquitin E3 ligase activity of BRCA1 and its biological functions
- PMID: 18179693
- PMCID: PMC2254412
- DOI: 10.1186/1747-1028-3-1
The ubiquitin E3 ligase activity of BRCA1 and its biological functions
Abstract
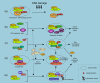
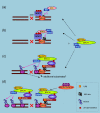
The basal-like breast cancer, a new category of breast cancer associated with poor prognosis and possibly unique chemosensitivity, is a current topic in the breast cancer field. Evidence from multiple sources strongly indicate that impairment of BRCA1 pathways is responsible for this phenotype, implying the importance of BRCA1 not only in familial breast cancers but also in sporadic cancers. BRCA1 acts as a hub protein that coordinates a diverse range of cellular pathways to maintain genomic stability. BRCA1 participates in multiple cellular supercomplexes to execute its tasks and, in most of the complexes, BRCA1 exists as a RING heterodimer with BARD1 to provide ubiquitin E3 ligase activity that is required for its tumor suppressor function. It was revealed recently that the BRCA1 RING finger is capable of catalyzing multiple types of ubiquitination depending upon the interacting E2, the ubiquitin carrier protein. BRCA1 may catalyze distinct ubiquitination on different substrates as the situation demands. On the other hand, in response to DNA double-strand breaks where BRCA1 plays its major role for homologous recombination repair, recent evidence showed that ubiquitination is a critical step to recruit BRCA1 to the damaged site through UIM (ubiquitin interacting motif) containing protein RAP80. Thus, ubiquitin and BRCA1 likely affect each other in many ways to perform cellular functions. Elucidation of this mechanism in relation to cell survival is now much anticipated because it could be a key to predict chemosensitivity of basal-like breast cancer.
Figures
References
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. - DOI - PubMed
-
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. - DOI - PMC - PubMed
-
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. - DOI - PubMed
-
- Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, Trudel M, Akslen LA. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. - PubMed
-
- Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, Bishop T, Benitez J, Rivas C, Bignon YJ, Chang-Claude J, Hamann U, Cornelisse CJ, Devilee P, Beckmann MW, Nestle-Kramling C, Daly PA, Haites N, Varley J, Lalloo F, Evans G, Maugard C, Meijers-Heijboer H, Klijn JG, Olah E, Gusterson BA, Pilotti S, Radice P, Scherneck S, Sobol H, Jacquemier J, Wagner T, Peto J, Stratton MR, McGuffog L, Easton DF. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11:5175–5180. doi: 10.1158/1078-0432.CCR-04-2424. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

