SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4
- PMID: 18179883
- PMCID: PMC2253972
- DOI: 10.1016/j.ajhg.2007.08.005
SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4
Abstract
The WNT-signaling pathway plays a major role during mammalian embryogenesis. We report a novel autosomal-recessive syndrome that consists of female to male sex reversal and renal, adrenal, and lung dysgenesis and is associated with additional developmental defects. Using a candidate-gene approach, we identified a disease-causing homozygous missense mutation in the human WNT4 gene. The mutation was found to result in markedly reduced WNT4 mRNA levels in vivo and in vitro and to downregulate WNT4-dependent inhibition of beta-catenin degradation. Taken together with previous observations in animal models, the present data attribute a pivotal role to WNT4 signaling during organogenesis in humans.
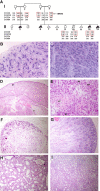
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

