Oncostatin M receptor-beta mutations underlie familial primary localized cutaneous amyloidosis
- PMID: 18179886
- PMCID: PMC2253984
- DOI: 10.1016/j.ajhg.2007.09.002
Oncostatin M receptor-beta mutations underlie familial primary localized cutaneous amyloidosis
Abstract
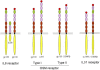
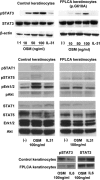
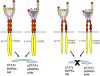
Familial primary localized cutaneous amyloidosis (FPLCA) is an autosomal-dominant disorder associated with chronic skin itching and deposition of epidermal keratin filament-associated amyloid material in the dermis. FPLCA has been mapped to 5p13.1-q11.2, and by candidate gene analysis, we identified missense mutations in the OSMR gene, encoding oncostatin M-specific receptor beta (OSMRbeta), in three families. OSMRbeta is a component of the oncostatin M (OSM) type II receptor and the interleukin (IL)-31 receptor, and cultured FPLCA keratinocytes showed reduced activation of Jak/STAT, MAPK, and PI3K/Akt pathways after OSM or IL-31 cytokine stimulation. The pathogenic amino acid substitutions are located within the extracellular fibronectin type III-like (FNIII) domains, regions critical for receptor dimerization and function. OSM and IL-31 signaling have been implicated in keratinocyte cell proliferation, differentiation, apoptosis, and inflammation, but our OSMR data in individuals with FPLCA represent the first human germline mutations in this cytokine receptor complex and provide new insight into mechanisms of skin itching.
Figures


References
-
- Breathnach S.M. Amyloid and the amyloidosis of the skin. In: Burns T., Breathnach S., Cox C., Griffiths C., editors. Rook's Textbook of Dermatology. Volume 3. Blackwell Scientific; Oxford: 2004. pp. 57.36–57.51.
-
- Kumakiri M., Hashimoto K. Histogenesis of primary localized cutaneous amyloidosis: sequential change of epidermal keratinocytes to amyloid via filamentous degeneration. J. Invest. Dermatol. 1979;73:150–162. - PubMed
-
- Kobayashi H., Hashimoto K. Amyloidogenesis in organ-limited cutaneous amyloidosis: an antigenic identity between epidermal keratin and skin amyloid. J. Invest. Dermatol. 1983;80:66–72. - PubMed
-
- Schepis C., Siragusa M., Gagliardi M.E., Torre V., Cicciarello R., Albiero F., Cavallari V. Primary macular amyloidosis: an ultrastructural approach to diagnosis. Ultrastruct. Pathol. 1999;23:279–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

