Disruption of neurexin 1 associated with autism spectrum disorder
- PMID: 18179900
- PMCID: PMC2253961
- DOI: 10.1016/j.ajhg.2007.09.011
Disruption of neurexin 1 associated with autism spectrum disorder
Abstract
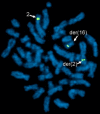
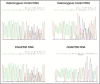
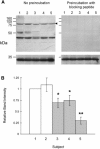
Autism is a neurodevelopmental disorder of complex etiology in which genetic factors play a major role. We have implicated the neurexin 1 (NRXN1) gene in two independent subjects who display an autism spectrum disorder (ASD) in association with a balanced chromosomal abnormality involving 2p16.3. In the first, with karyotype 46,XX,ins(16;2)(q22.1;p16.1p16.3)pat, NRXN1 is directly disrupted within intron 5. Importantly, the father possesses the same chromosomal abnormality in the absence of ASD, indicating that the interruption of alpha-NRXN1 is not fully penetrant and must interact with other factors to produce ASD. The breakpoint in the second subject, with 46,XY,t(1;2)(q31.3;p16.3)dn, occurs approximately 750 kb 5' to NRXN1 within a 2.6 Mb genomic segment that harbors no currently annotated genes. A scan of the NRXN1 coding sequence in a cohort of ASD subjects, relative to non-ASD controls, revealed that amino acid alterations in neurexin 1 are not present at high frequency in ASD. However, a number of rare sequence variants in the coding region, including two missense changes in conserved residues of the alpha-neurexin 1 leader sequence and of an epidermal growth factor (EGF)-like domain, respectively, suggest that even subtle changes in NRXN1 might contribute to susceptibility to ASD.
Figures



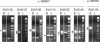
References
-
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveillance Summaries. 2007;56:12–28. - PubMed
-
- Santangelo S.L., Tsatsanis K. What is known about autism: Genes, brain, and behavior. Am. J. Pharmacogenomics. 2005;5:71–92. - PubMed
-
- Grice D.E., Buxbaum J.D. The genetics of autism spectrum disorders. Neuromolecular Med. 2006;8:451–460. - PubMed
-
- Xu J., Zwaigenbaum L., Szatmari P., Scherer S.W. Molecular cytogenetics of autism. Curr. Genomics. 2004;5:347–364.
-
- Vorstman J.A., Staal W.G., van Daalen E., van Engeland H., Hochstenbach P.F., Franke L. Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol. Psychiatry. 2006;11:18–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

