Mutation analysis of CHRNA1, CHRNB1, CHRND, and RAPSN genes in multiple pterygium syndrome/fetal akinesia patients
- PMID: 18179903
- PMCID: PMC2253973
- DOI: 10.1016/j.ajhg.2007.09.016
Mutation analysis of CHRNA1, CHRNB1, CHRND, and RAPSN genes in multiple pterygium syndrome/fetal akinesia patients
Abstract
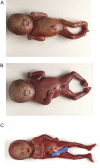
Multiple pterygium syndromes (MPS) comprise a group of multiple congenital anomaly disorders characterized by webbing (pterygia) of the neck, elbows, and/or knees and joint contractures (arthrogryposis). MPS are phenotypically and genetically heterogeneous but are traditionally divided into prenatally lethal and nonlethal (Escobar) types. Previously, we and others reported that recessive mutations in the embryonal acetylcholine receptor g subunit (CHRNG) can cause both lethal and nonlethal MPS, thus demonstrating that pterygia resulted from fetal akinesia. We hypothesized that mutations in acetylcholine receptor-related genes might also result in a MPS/fetal akinesia phenotype and so we analyzed 15 cases of lethal MPS/fetal akinesia without CHRNG mutations for mutations in the CHRNA1, CHRNB1, CHRND, and rapsyn (RAPSN) genes. No CHRNA1, CHRNB1, or CHRND mutations were detected, but a homozygous RAPSN frameshift mutation, c.1177-1178delAA, was identified in a family with three children affected with lethal fetal akinesia sequence. Previously, RAPSN mutations have been reported in congenital myasthenia. Functional studies were consistent with the hypothesis that whereas incomplete loss of rapsyn function may cause congenital myasthenia, more severe loss of function can result in a lethal fetal akinesia phenotype.
Figures


Similar articles
-
Germline mutation in DOK7 associated with fetal akinesia deformation sequence.J Med Genet. 2009 May;46(5):338-40. doi: 10.1136/jmg.2008.065425. Epub 2009 Mar 3. J Med Genet. 2009. PMID: 19261599
-
Acetylcholine receptor pathway mutations explain various fetal akinesia deformation sequence disorders.Am J Hum Genet. 2008 Feb;82(2):464-76. doi: 10.1016/j.ajhg.2007.11.006. Am J Hum Genet. 2008. PMID: 18252226 Free PMC article.
-
Mutations in the embryonal subunit of the acetylcholine receptor (CHRNG) cause lethal and Escobar variants of multiple pterygium syndrome.Am J Hum Genet. 2006 Aug;79(2):390-5. doi: 10.1086/506256. Epub 2006 Jun 20. Am J Hum Genet. 2006. PMID: 16826531 Free PMC article.
-
Prenatal diagnosis and genetic analysis of fetal akinesia deformation sequence and multiple pterygium syndrome associated with neuromuscular junction disorders: a review.Taiwan J Obstet Gynecol. 2012 Mar;51(1):12-7. doi: 10.1016/j.tjog.2012.01.004. Taiwan J Obstet Gynecol. 2012. PMID: 22482962 Review.
-
CHRNG-related nonlethal multiple pterygium syndrome: Muscle imaging pattern and clinical, histopathological, and molecular genetic findings.Am J Med Genet A. 2019 Jun;179(6):915-926. doi: 10.1002/ajmg.a.61122. Epub 2019 Mar 14. Am J Med Genet A. 2019. PMID: 30868735 Review.
Cited by
-
Ion Channel Gene Mutations Causing Skeletal Muscle Disorders: Pathomechanisms and Opportunities for Therapy.Cells. 2021 Jun 16;10(6):1521. doi: 10.3390/cells10061521. Cells. 2021. PMID: 34208776 Free PMC article. Review.
-
Lethal multiple pterygium syndrome, the extreme end of the RYR1 spectrum.BMC Musculoskelet Disord. 2016 Mar 1;17:109. doi: 10.1186/s12891-016-0947-5. BMC Musculoskelet Disord. 2016. PMID: 26932181 Free PMC article.
-
Prenatal Diagnosis of Lethal Multiple Pterygium Syndrome Using Two-and Three-Dimensional Ultrasonography.J Clin Imaging Sci. 2012;2:65. doi: 10.4103/2156-7514.103055. Epub 2012 Oct 31. J Clin Imaging Sci. 2012. PMID: 23230547 Free PMC article.
-
Autosomal-Dominant Multiple Pterygium Syndrome Is Caused by Mutations in MYH3.Am J Hum Genet. 2015 May 7;96(5):841-9. doi: 10.1016/j.ajhg.2015.04.004. Am J Hum Genet. 2015. PMID: 25957469 Free PMC article.
-
Germline mutations in RYR1 are associated with foetal akinesia deformation sequence/lethal multiple pterygium syndrome.Acta Neuropathol Commun. 2014 Dec 5;2:148. doi: 10.1186/s40478-014-0148-0. Acta Neuropathol Commun. 2014. PMID: 25476234 Free PMC article.
References
-
- Hall J.G., Reed S.D., Rosenbaum K.N., Gershanik J., Chen H., Wilson K.M. Limb pterygium syndromes: a review and report of eleven patients. Am. J. Med. Genet. 1982;12:377–379. - PubMed
-
- Hall J.G. The lethal multiple pterygium syndromes. Am. J. Med. Genet. 1984;4:803–807. - PubMed
-
- Froster U.G., Stallmach T., Wisser J., Hebisch G., Robbiani M.B., Huch R., Huch A. Lethal multiple pterygium syndrome: suggestion for a consistent pathological workup and review of reported cases. Am. J. Med. Genet. 1997;68:82–85. - PubMed
-
- Cox P.M., Brueton L.A., Bjelogrlic P., Pomroy P., Sewry C.A. Diversity of neuromuscular pathology in lethal multiple pterygium syndrome. Pediatr. Dev. Pathol. 2003;6:59–68. - PubMed
-
- Morgan N.V., Brueton L.A., Cox P., Greally M.T., Tolmie J., Pasha S., Aligianis I.A., van Bokhoven H., Marton T., Al-Gazali L. Mutations in the embryonal subunit of the acetylcholine receptor (CHRNG) cause lethal and Escobar variants of multiple pterygium syndrome. Am. J. Hum. Genet. 2006;79:390–395. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

