Evidence of a possible role for Lys-gamma3-MSH in the regulation of adipocyte function
- PMID: 18180326
- PMCID: PMC2216415
- DOI: 10.1677/JOE-07-0391
Evidence of a possible role for Lys-gamma3-MSH in the regulation of adipocyte function
Abstract
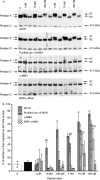
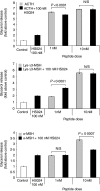
Lys-gamma3-MSH is a melanocortin peptide derived from the C-terminal of the 16 kDa fragment of POMC. The physiological role of Lys-gamma3-MSH is unclear, although it has previously been shown that, although not directly steroidogenic, it can act to potentiate the steroidogenic response of adrenal cortical cells to ACTH. This synergistic effect appears to be correlated with an ability to increase the activity of hormone sensitive lipase (HSL) and therefore the rate of cholesterol ester hydrolysis. Ligand binding studies have suggested that high-affinity binding sites for Lys-gamma3-MSH exist in the adrenal gland and a number of other rat tissues that express HSL, including adipose, skeletal muscle and testes. To investigate the hypothesis that Lys-gamma3-MSH may play a wider role in cholesterol and lipid metabolism, we tested the effect of Lys-gamma3-MSH on lipolysis, an HSL-mediated process, in 3T3-L1 adipocytes. In comparison with other melanocortin peptides, Lys-gamma3-MSH was found to be a potent stimulator of lipolysis. It was also able to phosphorylate HSL at key serine residues and stimulate the hyperphosphorylation of perilipin A. The receptor through which the lipolytic actions of Lys-gamma3-MSH are being mediated is not clear. Attempts to characterise this receptor suggest that either the pharmacology of the melanocortin receptor 5 in 3T3-L1 adipocytes is different from that described when expressed in heterologous systems or the possibility that a further, as yet uncharacterised, receptor exists.
Figures



References
-
- Al-Dujaili EA, Hope J, Estivariz FE, Lowry PJ, Edwards CR. Circulating human pituitary pro-gamma-melanotropin enhances the adrenal response to ACTH. Nature. 1981;29:156–159. - PubMed
-
- Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response isoproterenol and govern activation properties in vitro. Journal of Biological Chemistry. 1998;273:215–221. - PubMed
-
- Bennett HPJ. Use of ion-exchange Sep-Pak cartridges in the batch fractionation of pituitary peptides. Journal of Chromatography. 1986;359:383–390. - PubMed
-
- Bicknell AB, Lomthaisong K, Woods RJ, Hutchinson EG, Bennett HP, Gladwell RT, Lowry PJ. Characterisation of a serine protease that cleaves pro-gamma-melanotropin at the adrenal to stimulate growth. Cell. 2001;105:903–912. - PubMed
-
- Bohlen P, Esch F, Shibasaki T, Baird A, Ling N, Guillemin R. Isolation and characterization of γ1-melanotropin-like peptide from bovine neurointermediate pituitary. FEBS Letters. 1981;128:67–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

