Lethal mutagenesis of picornaviruses with N-6-modified purine nucleoside analogues
- PMID: 18180344
- PMCID: PMC2258490
- DOI: 10.1128/AAC.01056-07
Lethal mutagenesis of picornaviruses with N-6-modified purine nucleoside analogues
Abstract
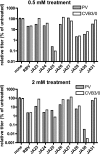
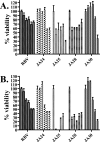
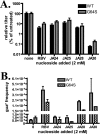
RNA viruses exhibit extraordinarily high mutation rates during genome replication. Nonnatural ribonucleosides that can increase the mutation rate of RNA viruses by acting as ambiguous substrates during replication have been explored as antiviral agents acting through lethal mutagenesis. We have synthesized novel N-6-substituted purine analogues with ambiguous incorporation characteristics due to tautomerization of the nucleobase. The most potent of these analogues reduced the titer of poliovirus (PV) and coxsackievirus (CVB3) over 1,000-fold during a single passage in HeLa cell culture, with an increase in transition mutation frequency up to 65-fold. Kinetic analysis of incorporation by the PV polymerase indicated that these analogues were templated ambiguously with increased efficiency compared to the known mutagenic nucleoside ribavirin. Notably, these nucleosides were not efficient substrates for cellular ribonucleotide reductase in vitro, suggesting that conversion to the deoxyriboucleoside may be hindered, potentially limiting genetic damage to the host cell. Furthermore, a high-fidelity PV variant (G64S) displayed resistance to the antiviral effect and mutagenic potential of these analogues. These purine nucleoside analogues represent promising lead compounds in the development of clinically useful antiviral therapies based on the strategy of lethal mutagenesis.
Figures

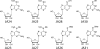


Similar articles
-
Mutational robustness of an RNA virus influences sensitivity to lethal mutagenesis.J Virol. 2012 Mar;86(5):2869-73. doi: 10.1128/JVI.05712-11. Epub 2011 Dec 21. J Virol. 2012. PMID: 22190724 Free PMC article.
-
Lethal mutagenesis of poliovirus mediated by a mutagenic pyrimidine analogue.J Virol. 2007 Oct;81(20):11256-66. doi: 10.1128/JVI.01028-07. Epub 2007 Aug 8. J Virol. 2007. PMID: 17686844 Free PMC article.
-
Extinction of Zika Virus and Usutu Virus by Lethal Mutagenesis Reveals Different Patterns of Sensitivity to Three Mutagenic Drugs.Antimicrob Agents Chemother. 2018 Aug 27;62(9):e00380-18. doi: 10.1128/AAC.00380-18. Print 2018 Sep. Antimicrob Agents Chemother. 2018. PMID: 29914957 Free PMC article.
-
Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity.Viruses. 2022 Apr 18;14(4):841. doi: 10.3390/v14040841. Viruses. 2022. PMID: 35458571 Free PMC article. Review.
-
Challenges for the development of ribonucleoside analogues as inducers of error catastrophe.Antivir Chem Chemother. 2004 Jan;15(1):1-13. doi: 10.1177/095632020401500101. Antivir Chem Chemother. 2004. PMID: 15074710 Review.
Cited by
-
Mutational robustness of an RNA virus influences sensitivity to lethal mutagenesis.J Virol. 2012 Mar;86(5):2869-73. doi: 10.1128/JVI.05712-11. Epub 2011 Dec 21. J Virol. 2012. PMID: 22190724 Free PMC article.
-
Lethal mutagenesis in viruses and bacteria.Genetics. 2009 Oct;183(2):639-50. doi: 10.1534/genetics.109.106492. Epub 2009 Jul 20. Genetics. 2009. PMID: 19620390 Free PMC article.
-
Therapeutic and prevention strategies against human enterovirus 71 infection.World J Virol. 2015 May 12;4(2):78-95. doi: 10.5501/wjv.v4.i2.78. World J Virol. 2015. PMID: 25964873 Free PMC article. Review.
-
Involvement of a joker mutation in a polymerase-independent lethal mutagenesis escape mechanism.Virology. 2016 Jul;494:257-66. doi: 10.1016/j.virol.2016.04.023. Epub 2016 Apr 29. Virology. 2016. PMID: 27136067 Free PMC article.
-
Inhibition of RNA recruitment and replication of an RNA virus by acridine derivatives with known anti-prion activities.PLoS One. 2009 Oct 13;4(10):e7376. doi: 10.1371/journal.pone.0007376. PLoS One. 2009. PMID: 19823675 Free PMC article.
References
-
- Arnold, J. J., and C. E. Cameron. 2000. Poliovirus RNA-dependent RNA polymerase (3D(pol)). Assembly of stable, elongation-competent complexes by using a symmetrical primer-template substrate (sym/sub). J. Biol. Chem. 275:5329-5336. - PubMed
-
- Brown, D. M., and P. K. Lin. 1991. Synthesis and duplex stability of oligonucleotides containing adenine-guanine analogues. Carbohydr. Res. 216:129-139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

