Increase of virulence and its phenotypic traits in drug-resistant strains of Candida albicans
- PMID: 18180350
- PMCID: PMC2258496
- DOI: 10.1128/AAC.01223-07
Increase of virulence and its phenotypic traits in drug-resistant strains of Candida albicans
Abstract
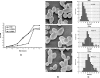
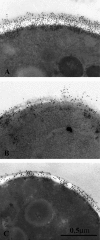
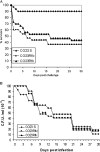
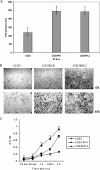
There is concern about the rise of antifungal drug resistance, but little is known about comparative biological properties and pathogenicity of drug-resistant strains. We generated fluconazole (FLC; CO23 RFLC)- or micafungin (FK; CO23 RFK)-resistant strains of Candida albicans by treating a FLC- and FK-susceptible strain of this fungus (CO23 S) with stepwise-increasing concentrations of either drug. Molecular analyses showed that CO23 RFLC had acquired markedly increased expression of the drug-resistance efflux pump encoded by the MDR1 gene, whereas CO23 RFK had a homozygous mutation in the FSK1 gene. These genetic modifications did not alter to any extent the growth capacity of the drug-resistant strains in vitro, either at 28 degrees C or at 37 degrees C, but markedly increased their experimental pathogenicity in a systemic mouse infection model, as assessed by the overall mortality and target organ invasion. Interestingly, no apparent increase in the vaginopathic potential of the strains was observed with an estrogen-dependent rat vaginal infection. The increased pathogenicity of drug-resistant strains for systemic infection was associated with a number of biochemical and physiological changes, including (i) marked cellular alterations associated with a different expression and content of major cell wall polysaccharides, (ii) more rapid and extensive hypha formation in both liquid and solid media, and (iii) increased adherence to plastic and a propensity for biofilm formation. Overall, our data demonstrate that experimentally induced resistance to antifungal drugs, irrespective of drug family, can substantially divert C. albicans biology, affecting in particular biological properties of potential relevance for deep-seated candidiasis.
Figures

Similar articles
-
Characterization of biofilms in drug-sensitive and drug-resistant strains of Candida albicans.J Chemother. 2013 Apr;25(2):87-95. doi: 10.1179/1973947812Y.0000000047. J Chemother. 2013. PMID: 23684356
-
Genotypic and phenotypic characterization of Candida albicans Lebanese hospital isolates resistant and sensitive to caspofungin.Fungal Genet Biol. 2019 Jun;127:12-22. doi: 10.1016/j.fgb.2019.02.008. Epub 2019 Feb 20. Fungal Genet Biol. 2019. PMID: 30794951
-
Molecular mechanism of fluconazole resistance and pathogenicity attributes of Lebanese Candida albicans hospital isolates.Fungal Genet Biol. 2021 Aug;153:103575. doi: 10.1016/j.fgb.2021.103575. Epub 2021 May 24. Fungal Genet Biol. 2021. PMID: 34033880
-
Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery.Int J Antimicrob Agents. 2014 May;43(5):395-402. doi: 10.1016/j.ijantimicag.2013.12.009. Epub 2014 Jan 22. Int J Antimicrob Agents. 2014. PMID: 24503221 Review.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
Cited by
-
Evaluation of Biofilm Formation in Candida tropicalis Using a Silicone-Based Platform with Synthetic Urine Medium.Microorganisms. 2020 May 1;8(5):660. doi: 10.3390/microorganisms8050660. Microorganisms. 2020. PMID: 32369936 Free PMC article.
-
Synergistic Effects and Mechanisms of Budesonide in Combination with Fluconazole against Resistant Candida albicans.PLoS One. 2016 Dec 22;11(12):e0168936. doi: 10.1371/journal.pone.0168936. eCollection 2016. PLoS One. 2016. PMID: 28006028 Free PMC article.
-
Glutathione metabolism in Candida albicans resistant strains to fluconazole and micafungin.PLoS One. 2014 Jun 4;9(6):e98387. doi: 10.1371/journal.pone.0098387. eCollection 2014. PLoS One. 2014. PMID: 24896636 Free PMC article.
-
The development of fluconazole resistance in Candida albicans - an example of microevolution of a fungal pathogen.J Microbiol. 2016 Mar;54(3):192-201. doi: 10.1007/s12275-016-5628-4. Epub 2016 Feb 27. J Microbiol. 2016. PMID: 26920879 Review.
-
Identification, typing, antifungal resistance profile, and biofilm formation of Candida albicans isolates from Lebanese hospital patients.Biomed Res Int. 2014;2014:931372. doi: 10.1155/2014/931372. Epub 2014 Jun 1. Biomed Res Int. 2014. PMID: 24982915 Free PMC article.
References
-
- Angiolella, L., B. Maras, A. R. Stringaro, G. Arancia, F. Mondello, A. Girolamo, A. T. Palamara, and A. Cassone. 2005. Glucan-associated protein modulations and ultrastructural changes of the cell wall in Candida albicans treated with micafungin, a water-soluble, lipopeptide antimycotic. J. Chemother. 17:409-416. - PubMed
-
- Bastert, J., M. Schaller, H. C. Korting, and E. G. V. Evans. 2001. Current and future approaches to antimycotic treatment in the era of resistant fungi and immunocompromised hosts. Int. J. Antimicrob. Agents 17:81-89. - PubMed
-
- Calabrese, D., J. Bille, and D. Sanglard. 2000. A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole. Microbiology 146:2743-2754. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

