Role for lysosomal enzyme beta-hexosaminidase in the control of mycobacteria infection
- PMID: 18180457
- PMCID: PMC2206601
- DOI: 10.1073/pnas.0708110105
Role for lysosomal enzyme beta-hexosaminidase in the control of mycobacteria infection
Abstract
The pathogenic mycobacteria that cause tuberculosis (TB) and TB-like diseases in humans and animals elude sterilizing immunity by residing within an intracellular niche in host macrophages, where they are protected from microbicidal attack. Recent studies have emphasized microbial mechanisms for evasion of host defense; less is known about mycobactericidal mechanisms that remain intact during initial infection. To better understand macrophage mechanisms for restricting mycobacteria growth, we examined Mycobacterium marinum infection of Drosophila S2 cells. Among approximately 1,000 host genes examined by RNAi depletion, the lysosomal enzyme beta-hexosaminidase was identified as an important factor in the control of mycobacterial infection. The importance of beta-hexosaminidase for restricting mycobacterial growth during mammalian infections was confirmed in macrophages from beta-hexosaminidase knockout mice. Beta-hexosaminidase was characterized as a peptidoglycan hydrolase that surprisingly exerts its mycobactericidal effect at the macrophage plasma membrane during mycobacteria-induced secretion of lysosomes. Thus, secretion of lysosomal enzymes is a mycobactericidal mechanism that may have a more general role in host defense.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
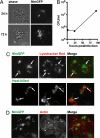
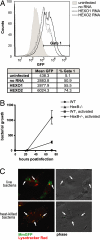
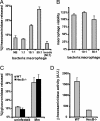
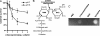

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

