Structural impact of three Parkinsonism-associated missense mutations on human DJ-1
- PMID: 18181649
- PMCID: PMC2657723
- DOI: 10.1021/bi701189c
Structural impact of three Parkinsonism-associated missense mutations on human DJ-1
Abstract
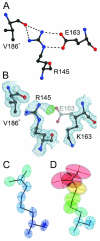
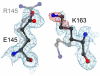
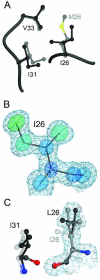
A number of missense mutations in the oxidative stress response protein DJ-1 are implicated in rare forms of familial Parkinsonism. The best-characterized Parkinsonian DJ-1 missense mutation, L166P, disrupts homodimerization and results in a poorly folded protein. The molecular basis by which the other Parkinsonism-associated mutations disrupt the function of DJ-1, however, is incompletely understood. In this study we show that three different Parkinsonism-associated DJ-1 missense mutations (A104T, E163K, and M26I) reduce the thermal stability of DJ-1 in solution by subtly perturbing the structure of DJ-1 without causing major folding defects or loss of dimerization. Atomic resolution X-ray crystallography shows that the A104T substitution introduces water and a discretely disordered residue into the core of the protein, E163K disrupts a key salt bridge with R145, and M26I causes packing defects in the core of the dimer. The deleterious effect of each Parkinsonism-associated mutation on DJ-1 is dissected by analysis of engineered substitutions (M26L, A104V, and E163K/R145E) that partially alleviate each of the defects introduced by the A104T, E163K and M26I mutations. In total, our results suggest that the protective function of DJ-1 can be compromised by diverse perturbations in its structural integrity, particularly near the junctions of secondary structural elements.
Figures





References
-
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
-
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 1997;231:509–513. - PubMed
-
- Lucas JI, Marin I. A New Evolutionary Paradigm for the Parkinson Disease Gene DJ-1. Mol. Biol. Evol. 2006;24:551–561. - PubMed
-
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA. 2004;101:9103–9108. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

