Progenitor cells and vascular disease
- PMID: 18181954
- PMCID: PMC6496281
- DOI: 10.1111/j.1365-2184.2008.00488.x
Progenitor cells and vascular disease
Abstract
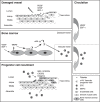
Vascular progenitor cells have been the focus of much attention in recent years; both from the point of view of their pathophysiological roles and their potential as therapeutic agents. However, there is as yet no definitive description of either endothelial or vascular smooth muscle progenitor cells. Cells with the ability to differentiate into mature endothelial and vascular smooth muscle reportedly reside within a number of different tissues, including bone marrow, spleen, cardiac muscle, skeletal muscle and adipose tissue. Within these niches, vascular progenitor cells remain quiescent, until mobilized in response to injury or disease. Once mobilized, these progenitor cells enter the circulation and migrate to sites of damage, where they contribute to the remodelling process. It is generally perceived that endothelial progenitors are reparative, acting to restore vascular homeostasis, while smooth muscle progenitors contribute to pathological changes. Indeed, the number of circulating endothelial progenitor cells inversely correlates with exposure to cardiovascular risk factors and numbers of animal models and human studies have demonstrated therapeutic roles for endothelial progenitor cells, which can be enhanced by manipulating them to overexpress vasculo-protective genes. It remains to be determined whether smooth muscle progenitor cells, which are less well studied than their endothelial counterparts, can likewise be manipulated to achieve therapeutic benefit. This review outlines our current understanding of endothelial and smooth muscle progenitor cell biology, their roles in vascular disease and their potential as therapeutic agents.
Figures
Similar articles
-
Progenitor cells in vascular disease.J Cell Mol Med. 2005 Jul-Sep;9(3):583-91. doi: 10.1111/j.1582-4934.2005.tb00490.x. J Cell Mol Med. 2005. PMID: 16202207 Free PMC article. Review.
-
Progenitor cells in vascular repair.Curr Opin Lipidol. 2007 Oct;18(5):534-9. doi: 10.1097/MOL.0b013e3282a66082. Curr Opin Lipidol. 2007. PMID: 17885424 Review.
-
Smooth muscle progenitor cells: friend or foe in vascular disease?Curr Stem Cell Res Ther. 2009 May;4(2):131-40. doi: 10.2174/157488809788167454. Curr Stem Cell Res Ther. 2009. PMID: 19442197 Free PMC article. Review.
-
Ambivalence of progenitor cells in vascular repair and plaque stability.Curr Opin Lipidol. 2008 Oct;19(5):491-7. doi: 10.1097/MOL.0b013e32830dfe33. Curr Opin Lipidol. 2008. PMID: 18769230 Review.
-
Progenitor cell-derived smooth muscle cells in vascular disease.Biochem Pharmacol. 2010 Jun 15;79(12):1706-13. doi: 10.1016/j.bcp.2010.01.027. Epub 2010 Feb 1. Biochem Pharmacol. 2010. PMID: 20117099 Review.
Cited by
-
Differentiation of smooth muscle progenitor cells in peripheral blood and its application in tissue engineered blood vessels.J Zhejiang Univ Sci B. 2008 Dec;9(12):923-30. doi: 10.1631/jzus.B0820257. J Zhejiang Univ Sci B. 2008. PMID: 19067459 Free PMC article.
-
The role of metal components in the cardiovascular effects of PM2.5.PLoS One. 2013 Dec 27;8(12):e83782. doi: 10.1371/journal.pone.0083782. eCollection 2013. PLoS One. 2013. PMID: 24386277 Free PMC article.
-
Mechanism of arterial remodeling in chronic allograft vasculopathy.Front Med. 2011 Sep;5(3):248-53. doi: 10.1007/s11684-011-0149-3. Epub 2011 Oct 2. Front Med. 2011. PMID: 21964706 Review.
-
Vascular extracellular matrix and arterial mechanics.Physiol Rev. 2009 Jul;89(3):957-89. doi: 10.1152/physrev.00041.2008. Physiol Rev. 2009. PMID: 19584318 Free PMC article. Review.
-
Remodeling of the thoracic aorta after bone marrow cell transplantation.Int J Clin Exp Pathol. 2014 Aug 15;7(9):5527-37. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337194 Free PMC article.
References
-
- Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, Tarnok A, Gielen S, Emmrich F, Schuler G, Hambrecht R (2004) Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise‐induced ischemia. Arterioscler. Thromb. Vasc. Biol. 24, 684–690. - PubMed
-
- Aicher A, Heeschen C, Dimmeler S (2004) The role of NOS3 in stem cell mobilization. Trends Mol. Med. 10, 421–425. - PubMed
-
- Asahara T, Kawamoto A (2004) Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol. Cell Physiol. 287, C572–C579. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
