EndoS from Streptococcus pyogenes is hydrolyzed by the cysteine proteinase SpeB and requires glutamic acid 235 and tryptophans for IgG glycan-hydrolyzing activity
- PMID: 18182097
- PMCID: PMC2266755
- DOI: 10.1186/1471-2180-8-3
EndoS from Streptococcus pyogenes is hydrolyzed by the cysteine proteinase SpeB and requires glutamic acid 235 and tryptophans for IgG glycan-hydrolyzing activity
Abstract
Background: The endoglycosidase EndoS and the cysteine proteinase SpeB from the human pathogen Streptococcus pyogenes are functionally related in that they both hydrolyze IgG leading to impairment of opsonizing antibodies and thus enhance bacterial survival in human blood. In this study, we further investigated the relationship between EndoS and SpeB by examining their in vitro temporal production and stability and activity of EndoS. Furthermore, theoretical structure modeling of EndoS combined with site-directed mutagenesis and chemical blocking of amino acids was used to identify amino acids required for the IgG glycan-hydrolyzing activity of EndoS.
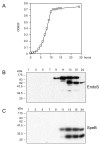
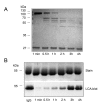
Results: We could show that during growth in vitro S. pyogenes secretes the IgG glycan-hydrolyzing endoglycosidase EndoS prior to the cysteine proteinase SpeB. Upon maturation SpeB hydrolyzes EndoS that then loses its IgG glycan-hydrolyzing activity. Sequence analysis and structural homology modeling of EndoS provided a basis for further analysis of the prerequisites for IgG glycan-hydrolysis. Site-directed mutagenesis and chemical modification of amino acids revealed that glutamic acid 235 is an essential catalytic residue, and that tryptophan residues, but not the abundant lysine or the single cysteine residues, are important for EndoS activity.
Conclusion: We present novel information about the amino acid requirements for IgG glycan-hydrolyzing activity of the immunomodulating enzyme EndoS. Furthermore, we show that the cysteine proteinase SpeB processes/degrades EndoS and thus emphasize the importance of the SpeB as a degrading/processing enzyme of proteins from the bacterium itself.
Figures



References
-
- Åkesson P, Rasmussen M, Mascini E, von Pawel-Rammingen U, Janulczyk R, Collin M, Olsén A, Mattsson E, Olsson ML, Björck L, Christensson B. Low antibody levels against cell wall-attached proteins of Streptococcus pyogenes predispose for severe invasive disease. Journal of Infectious Diseases. 2004;189:797–804. doi: 10.1086/381982. - DOI - PubMed
-
- Voyich JM, Sturdevant DE, Braughton KR, Kobayashi SD, Lei B, Virtaneva K, Dorward DW, Musser JM, DeLeo FR. Genome-wide protective response used by group A Streptococcus to evade destruction by human polymorphonuclear leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1996–2001. doi: 10.1073/pnas.0337370100. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

