Evolution of GITRL immune function: murine GITRL exhibits unique structural and biochemical properties within the TNF superfamily
- PMID: 18182486
- PMCID: PMC2206588
- DOI: 10.1073/pnas.0710529105
Evolution of GITRL immune function: murine GITRL exhibits unique structural and biochemical properties within the TNF superfamily
Abstract
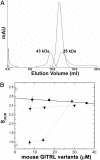
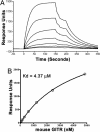
Glucocorticoid-induced TNF receptor ligand (GITRL), a recently identified member of the TNF superfamily, binds to its receptor, GITR, on both effector and regulatory T cells and generates positive costimulatory signals implicated in a wide range of T cell functions. In contrast to all previously characterized homotrimeric TNF family members, the mouse GITRL crystal structure reveals a previously unrecognized dimeric assembly that is stabilized via a unique "domain-swapping" interaction. Consistent with its crystal structure, mouse GITRL exists as a stable dimer in solution. Structure-guided mutagenesis studies confirmed the determinants responsible for dimerization and support a previously unrecognized receptor-recognition surface for mouse GITRL that has not been observed for any other TNF family members. Taken together, the unique structural and biochemical behavior of mouse GITRL, along with the unusual domain organization of murine GITR, support a previously unrecognized mechanism for signaling within the TNF superfamily.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


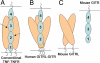
Similar articles
-
Atypical TNF-TNFR superfamily binding interface in the GITR-GITRL complex for T cell activation.Cell Rep. 2021 Sep 21;36(12):109734. doi: 10.1016/j.celrep.2021.109734. Cell Rep. 2021. PMID: 34551288
-
Assembly and structural properties of glucocorticoid-induced TNF receptor ligand: Implications for function.Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19452-7. doi: 10.1073/pnas.0709264104. Epub 2007 Nov 26. Proc Natl Acad Sci U S A. 2007. PMID: 18040044 Free PMC article.
-
Structural basis for ligand-mediated mouse GITR activation.Proc Natl Acad Sci U S A. 2008 Jan 15;105(2):641-5. doi: 10.1073/pnas.0711206105. Epub 2008 Jan 4. Proc Natl Acad Sci U S A. 2008. PMID: 18178614 Free PMC article.
-
GITR/GITRL: more than an effector T cell co-stimulatory system.Eur J Immunol. 2007 May;37(5):1165-9. doi: 10.1002/eji.200636933. Eur J Immunol. 2007. PMID: 17407102 Review.
-
Glucocorticoid-induced TNFR-related (GITR) protein and its ligand in antitumor immunity: functional role and therapeutic modulation.Clin Dev Immunol. 2010;2010:239083. doi: 10.1155/2010/239083. Epub 2010 Sep 26. Clin Dev Immunol. 2010. PMID: 20936139 Free PMC article. Review.
Cited by
-
Anti-glucocorticoid-induced Tumor Necrosis Factor-Related Protein (GITR) Therapy Overcomes Radiation-Induced Treg Immunosuppression and Drives Abscopal Effects.Front Immunol. 2018 Sep 20;9:2170. doi: 10.3389/fimmu.2018.02170. eCollection 2018. Front Immunol. 2018. PMID: 30294332 Free PMC article.
-
Development of a fully human anti-GITR antibody with potent antitumor activity using H2L2 mice.FEBS Open Bio. 2022 Aug;12(8):1542-1557. doi: 10.1002/2211-5463.13451. Epub 2022 Jun 21. FEBS Open Bio. 2022. PMID: 35674216 Free PMC article.
-
Analysis of domain-swapped oligomers reveals local sequence preferences and structural imprints at the linker regions and swapped interfaces.PLoS One. 2012;7(7):e39305. doi: 10.1371/journal.pone.0039305. Epub 2012 Jul 27. PLoS One. 2012. PMID: 22848353 Free PMC article.
-
Optimal target saturation of ligand-blocking anti-GITR antibody IBI37G5 dictates FcγR-independent GITR agonism and antitumor activity.Cell Rep Med. 2022 Jun 21;3(6):100660. doi: 10.1016/j.xcrm.2022.100660. Cell Rep Med. 2022. PMID: 35732156 Free PMC article.
-
Modulation of GITR for cancer immunotherapy.Curr Opin Immunol. 2012 Apr;24(2):217-24. doi: 10.1016/j.coi.2011.12.011. Epub 2012 Jan 12. Curr Opin Immunol. 2012. PMID: 22245556 Free PMC article. Review.
References
-
- Locksley RM, Killeen N, Lenardo MJ. Cell. 2001;104:487–501. - PubMed
-
- Bodmer JL, Schneider P, Tschopp J. Trends Biochem Sci. 2002;27:19–26. - PubMed
-
- Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. Eur J Immunol. 2007;37:1165–1169. - PubMed
-
- Shevach EM, Stephens GL. Nat Rev Immunol. 2006;6:613–618. - PubMed
-
- Watts TH. Annu Rev Immunol. 2005;23:23–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

