Ecological rules governing helminth-microparasite coinfection
- PMID: 18182496
- PMCID: PMC2206576
- DOI: 10.1073/pnas.0707221105
Ecological rules governing helminth-microparasite coinfection
Abstract
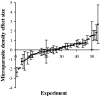
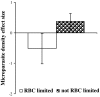
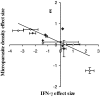
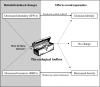
Coinfection of a host by multiple parasite species has important epidemiological and clinical implications. However, the direction and magnitude of effects vary considerably among systems, and, until now, there has been no general framework within which to explain this variation. Community ecology has great potential for application to such problems in biomedicine. Here, metaanalysis of data from 54 experiments on laboratory mice reveals that basic ecological rules govern the outcome of coinfection across a broad spectrum of parasite taxa. Specifically, resource-based ("bottom-up") and predator-based ("top-down") control mechanisms combined to determine microparasite population size in helminth-coinfected hosts. Coinfection imposed bottom-up control (resulting in decreased microparasite density) when a helminth that causes anemia was paired with a microparasite species that requires host red blood cells. At the same time, coinfection impaired top-down control of microparasites by the immune system: the greater the helminth-induced suppression of the inflammatory cytokine interferon (IFN)-gamma, the greater the increase in microparasite density. These results suggest that microparasite population growth will be most explosive when underlying helminths do not impose resource limitations but do strongly modulate IFN-gamma responses. Surprisingly simple rules and an ecological framework within which to analyze biomedical data thus emerge from analysis of this dataset. Through such an interdisciplinary lens, predicting the outcome of coinfection may become tractable.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

