Zebrafish in hematology: sushi or science?
- PMID: 18182572
- PMCID: PMC2275003
- DOI: 10.1182/blood-2007-10-052761
Zebrafish in hematology: sushi or science?
Abstract
After a decade of the "modern era" of zebrafish hematology research, what have been their major contributions to hematology and what challenges does the model face? This review argues that, in hematology, zebrafish have demonstrated their suitability, are proving their utility, have supplied timely and novel discoveries, and are poised for further significant contributions. It presents an overview of the anatomy, physiology, and genetics of zebrafish hematopoiesis underpinning their use in hematology research. Whereas reverse genetic techniques enable functional studies of particular genes of interest, forward genetics remains zebrafish's particular strength. Mutants with diverse and interesting hematopoietic defects are emerging from multiple genetic screens. Some mutants model hereditary blood diseases, occasionally leading to disease genes first; others provide insights into developmental hematology. Models of malignant hematologic disorders provide tools for drug-target and pharmaceutics discovery. Numerous transgenic zebrafish with fluorescently marked blood cells enable live-cell imaging of inflammatory responses and host-pathogen interactions previously inaccessible to direct observation in vivo, revealing unexpected aspects of leukocyte behavior. Zebrafish disease models almost uniquely provide a basis for efficient whole animal chemical library screens for new therapeutics. Despite some limitations and challenges, their successes and discovery potential mean that zebrafish are here to stay in hematology research.
Figures

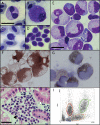

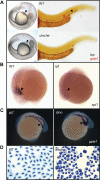
References
-
- Roosen-Runge E. Observations of the early development of the zebra fish, Brachydanio rerio. Anat Rec. 1937;70(Suppl 1):103.
-
- Laale HW. The biology and use of zebrafish, Brachydanio rerio, in fisheries research: a literature review. J Fish Biol. 1977;10:121–173.
-
- Colle-Vandevelde A. Blood anlage in Teleosti. Nature. 1963;198:1223. - PubMed
-
- Rieb J-P. La Circulation Sanguine Chez L'Embryon De Brachydanio Rerio. Ann Embryol Morphogenese. 1973;6:43–54.
-
- Johnson RA, Hart NH, Powell D. Studies on the morphology of blood cells in the fresh water teleost, Brachydanio rerio. Bull N Y Acad Sci. 1973;18:20–21.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

