Donor cell-derived osteopoiesis originates from a self-renewing stem cell with a limited regenerative contribution after transplantation
- PMID: 18182575
- PMCID: PMC2288731
- DOI: 10.1182/blood-2007-10-115725
Donor cell-derived osteopoiesis originates from a self-renewing stem cell with a limited regenerative contribution after transplantation
Abstract
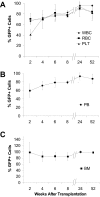
In principle, bone marrow transplantation should offer effective treatment for disorders originating from defects in mesenchymal stem cells. Results with the bone disease osteogenesis imperfecta support this hypothesis, although the rate of clinical improvement seen early after transplantation does not persist long term, raising questions as to the regenerative capacity of the donor-derived mesenchymal progenitors. We therefore studied the kinetics and histologic/anatomic pattern of osteopoietic engraftment after transplantation of GFP-expressing nonadherent marrow cells in mice. Serial tracking of donor-derived GFP(+) cells over 52 weeks showed abundant clusters of donor-derived osteoblasts/osteocytes in the epiphysis and metaphysis but not the diaphysis, a distribution that paralleled the sites of initial hematopoietic engraftment. Osteopoietic chimerism decreased from approximately 30% to 10% by 24 weeks after transplantation, declining to negligible levels thereafter. Secondary transplantation studies provided evidence for a self-renewing osteopoietic stem cell in the marrow graft. We conclude that a transplantable, primitive, self-renewing osteopoietic cell within the nonadherent marrow cell population engrafts in an endosteal niche, like hematopoietic stem cells, and regenerates a significant fraction of all bone cells. The lack of durable donor-derived osteopoiesis may reflect an intrinsic genetic program or exogenous environmental signaling that suppresses the differentiation capacity of the donor stem cells.
Figures





Comment in
-
Osteopoietic stem cells: transplantable, but regeneratively limited.Blood. 2008 Apr 15;111(8):3917-8. doi: 10.1182/blood-2008-02-135400. Blood. 2008. PMID: 18434966
References
-
- Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. - PubMed
-
- Brazelton TR, Nystrom M, Blau HM. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev Biol. 2003;262:64–74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

