NFAT but not NF-kappaB is critical for transcriptional induction of the prosurvival gene A1 after IgE receptor activation in mast cells
- PMID: 18182578
- PMCID: PMC2265451
- DOI: 10.1182/blood-2006-10-053371
NFAT but not NF-kappaB is critical for transcriptional induction of the prosurvival gene A1 after IgE receptor activation in mast cells
Abstract
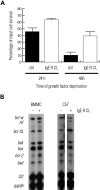
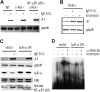
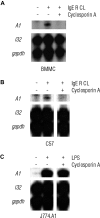
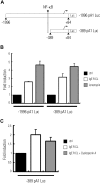
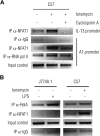
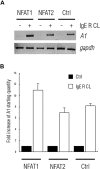
FcepsilonRI-activation-induced survival of mast cells is dependent on the expression and function of the prosurvival protein A1. The expression of A1 in lymphocytes and monocytes has previously been described to be transcriptionally regulated by NF-kappaB. Here we demonstrate that the expression of A1 in mast cells is not dependent on NF-kappaB but that NFAT plays a crucial role. FcepsilonRI-induced A1 expression was not affected in mast cells overexpressing an IkappaB-alpha super-repressor or cells lacking NF-kappaB subunits RelA, c-Rel, or c-Rel plus NF-kappaB1 p50. In contrast, inhibition of calcineurin and NFAT by cyclosporin A abrogated the expression of A1 in mast cells on FcepsilonRI-activation but had no effect on lipopolysaccharide-induced expression of A1 in J774A.1 monocytic cells. Cyclosporin A also inhibited luciferase expression in an A1 promoter reporter assay. A putative NFAT binding site in the A1 promoter showed inducible protein binding after FcepsilonRI crosslinking or treatment with ionomycin as detected in a band shift assay or chromatin immunoprecipitation. The binding protein was identified as NFAT1. Finally, mast cells expressing constitutively active NFAT1 exhibit increased expression of A1 after FcepsilonRI-stimulation. These results indicate that, in FcepsilonRI stimulated mast cells, A1 is transcriptionally regulated by NFAT1 but not by NF-kappaB.
Figures

Similar articles
-
Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation.J Exp Med. 2001 Dec 3;194(11):1561-69. doi: 10.1084/jem.194.11.1561. J Exp Med. 2001. PMID: 11733571 Free PMC article.
-
FcepsilonRI aggregation promotes survival of connective tissue-like mast cells but not mucosal-like mast cells.J Immunol. 2007 Apr 1;178(7):4177-83. doi: 10.4049/jimmunol.178.7.4177. J Immunol. 2007. PMID: 17371974
-
EphA2 stimulates VCAM-1 expression through calcium-dependent NFAT1 activity.Cell Signal. 2018 Sep;49:30-38. doi: 10.1016/j.cellsig.2018.05.008. Epub 2018 May 21. Cell Signal. 2018. PMID: 29793020 Free PMC article.
-
IgE-receptor activation of mast cells regulates phosphorylation and expression of forkhead and Bcl-2 family members.Scand J Immunol. 2006 Jan;63(1):1-6. doi: 10.1111/j.1365-3083.2006.01704.x. Scand J Immunol. 2006. PMID: 16398695
-
Fc epsilon RI-mediated induction of nuclear factor of activated T-cells.J Biol Chem. 1995 Jul 7;270(27):16333-8. doi: 10.1074/jbc.270.27.16333. J Biol Chem. 1995. PMID: 7608202
Cited by
-
Transcriptional Regulation of Mouse Mast Cell Differentiation and the Role of Human Lung Mast Cells in Airway Inflammation.Immunol Rev. 2025 May;331(1):e70026. doi: 10.1111/imr.70026. Immunol Rev. 2025. PMID: 40211768 Review.
-
Cell cycle and apoptosis regulation by NFAT transcription factors: new roles for an old player.Cell Death Dis. 2016 Apr 21;7(4):e2199. doi: 10.1038/cddis.2016.97. Cell Death Dis. 2016. PMID: 27100893 Free PMC article. Review.
-
Calcineurin-NFAT signalling in myeloid leucocytes: new prospects and pitfalls in immunosuppressive therapy.EMBO Mol Med. 2017 Aug;9(8):990-999. doi: 10.15252/emmm.201707698. EMBO Mol Med. 2017. PMID: 28606994 Free PMC article. Review.
-
NFATC2 Modulates Radiation Sensitivity in Dermal Fibroblasts From Patients With Severe Side Effects of Radiotherapy.Front Oncol. 2020 Dec 16;10:589168. doi: 10.3389/fonc.2020.589168. eCollection 2020. Front Oncol. 2020. PMID: 33392083 Free PMC article.
-
Targeting Mast Cells in Allergic Disease: Current Therapies and Drug Repurposing.Cells. 2022 Sep 27;11(19):3031. doi: 10.3390/cells11193031. Cells. 2022. PMID: 36230993 Free PMC article. Review.
References
-
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. - PubMed
-
- Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. - PubMed
-
- Nilsson G, Costa JJ, Metcalfe DD. Mast cells and basophils. In: Gallin JI SR, editor. Inflammation: Basic Principles and Clinical Correlates. ed 3. Philadelphia, PA: Lippincott-Raven; 1999. pp. 97–117.
-
- Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. - PubMed
-
- Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

