Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys
- PMID: 18182616
- PMCID: PMC2704336
- DOI: 10.1093/jnci/djm288
Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys
Abstract
Background: Patients with Birt-Hogg-Dubé (BHD) syndrome harbor germline mutations in the BHD tumor suppressor gene that are associated with an increased risk for kidney cancer. BHD encodes folliculin, a protein that may interact with the energy- and nutrient-sensing 5'-AMP-activated protein kinase-mammalian target of rapamycin (AMPK-mTOR) signaling pathways.
Methods: We used recombineering methods to generate mice with a conditional BHD allele and introduced the cadherin 16 (KSP)-Cre transgene to target BHD inactivation to the kidney. Kidney cell proliferation was measured by BrdU incorporation and phospho-histone H3 staining. Kidney weight data were analyzed with Wilcoxon's rank-sum, Student's t, and Welch's t tests. Hematoxylin and eosin staining and immunoblot analysis and immunohistochemistry of cell cycle and signaling proteins were performed on mouse kidney cells and tissues. BHD knockout mice and kidney cells isolated from BHD knockout and control mice were treated with the mTOR inhibitor rapamycin. Mouse survival was evaluated by Kaplan-Meier analyses. All statistical tests were two-sided.
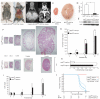
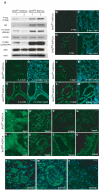
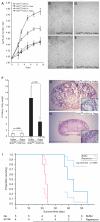
Results: BHD knockout mice developed enlarged polycystic kidneys and died from renal failure by 3 weeks of age. Targeted BHD knockout led to the activation of Raf-extracellular signal-regulated protein kinase (Erk)1/2 and Akt-mTOR pathways in the kidneys and increased expression of cell cycle proteins and cell proliferation. Rapamycin-treated BHD knockout mice had smaller kidneys than buffer-treated BHD knockout mice had (n = 4-6 mice per group, relative kidney/body weight ratios, mean = 4.64% vs 12.2%, difference = 7.6%, 95% confidence interval = 5.2% to 10.0%; P < .001) and longer median survival time (n = 4-5 mice per group, 41.5 vs 23 days; P = .0065 ).
Conclusions: Homozygous loss of BHD may initiate renal tumorigenesis in the mouse. The conditional BHD knockout mouse may be a useful research model for dissecting multistep kidney carcinogenesis, and rapamycin may be considered as a potential treatment for Birt-Hogg-Dubé syndrome.
Figures
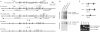



References
-
- Birt AR, Hogg GR, Dube WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–7. - PubMed
-
- Toro JR, Glenn GM, Duray PH, Darling T, Weirich G, Zbar B, et al. Birt-Hogg-Dube Syndrome: A Novel Marker of Kidney Neoplasia. Arch Dermatol. 1999;135:1195–1202. - PubMed
-
- Zbar B, Alvord WG, Glenn GM, Turner M, Pavlovich CP, Schmidt L, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dube syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. - PubMed
-
- Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn GM, Turner ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell. 2002;2:157–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

