Cetuximab potentiates oxaliplatin cytotoxic effect through a defect in NER and DNA replication initiation
- PMID: 18182978
- PMCID: PMC2359709
- DOI: 10.1038/sj.bjc.6604134
Cetuximab potentiates oxaliplatin cytotoxic effect through a defect in NER and DNA replication initiation
Abstract
Preclinical studies have demonstrated that the chemotherapeutic action of oxaliplatin, a third generation platinum derivative, is improved when combined with cetuximab, a monoclonal antibody inhibitor of epidermal growth factor receptors. To explore the mechanism of this synergistic benefit, we used HCT-8 and HCT-116, two human colon cancer cell lines, respectively, responsive and non-responsive to the oxaliplatin/cetuximab combination. We examined the effect of drug exposure on glutathione-S-transferase-mediated oxaliplatin detoxification, DNA-platinum adducts formation, cell cycle distribution, apoptosis, and the expression of multiple targets involved in DNA replication, recombination, and repair. The major changes we found in HCT-8 were a stimulation of oxaliplatin-DNA adduct formation associated with reduced expression of the key enzyme (excision repair cross complementation group1: ERCC1) in the key repair process of oxaliplatin-DNA platinum adduct, the nucleotide excision repair (NER), both at the mRNA and protein levels. We also observed a reduced expression of factors involved in DNA replication initiation, which correlates with an enrichment of cells in the G1 phase of the cell cycle as well as an acceleration of apoptosis. None of these changes occurred in the non-responsive HCT-116 cell that we used as a negative control. These findings support the fact that cetuximab potentiates the oxaliplatin-mediated cytotoxic effect as the result of inhibition of NER and also DNA replication initiation.
Figures
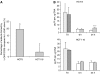
 ) or combined with cetuximab (C225,
) or combined with cetuximab (C225,  ) and followed after medium change by either vehicle (□); or 20 μg ml−1 cetuximab (
) and followed after medium change by either vehicle (□); or 20 μg ml−1 cetuximab ( ) exposure for 6 and 24 h (n=6). Dilution effect of proliferation on the DNA adduct content was corrected by [3H]-thymidine incorporation. Values are mean±s.e.m. of at least three independent experiments. ***P<0.005; *P<0.05. Student's t-test compared to oxaliplatin alone.
) exposure for 6 and 24 h (n=6). Dilution effect of proliferation on the DNA adduct content was corrected by [3H]-thymidine incorporation. Values are mean±s.e.m. of at least three independent experiments. ***P<0.005; *P<0.05. Student's t-test compared to oxaliplatin alone.
 ), or a combination of both (▪) for 6 and 24 h. Results are expressed as the mean±s.e.m. of three independent experiments. (B) Western blot analysis of ERCC1 protein expression after 24 h exposure to 5 μ
), or a combination of both (▪) for 6 and 24 h. Results are expressed as the mean±s.e.m. of three independent experiments. (B) Western blot analysis of ERCC1 protein expression after 24 h exposure to 5 μ
 ), 20 μg ml−1 cetuximab (C225,
), 20 μg ml−1 cetuximab (C225,  ), 5 μ
), 5 μ ), or a combination of both (
), or a combination of both ( ). (A representative experiment of three independent assessments is shown), and (B) kinetic evolution until 72 h of the percent of cells in G0/G1, S, and G2/M phases after exposure to either vehicle (control), 20 μg ml−1 cetuximab daily (C225), 5 μ
). (A representative experiment of three independent assessments is shown), and (B) kinetic evolution until 72 h of the percent of cells in G0/G1, S, and G2/M phases after exposure to either vehicle (control), 20 μg ml−1 cetuximab daily (C225), 5 μ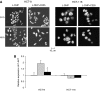
 ), or a combination of both (▪) compared to untreated cells. Results are expressed as the mean±s.e.m. of three independent experiments. *P<0.05. Student's t-test compared to the effect of oxaliplatin alone.
), or a combination of both (▪) compared to untreated cells. Results are expressed as the mean±s.e.m. of three independent experiments. *P<0.05. Student's t-test compared to the effect of oxaliplatin alone.
 ) and increased effect of oxaliplatin by more than 30% (▪) when combined with cetuximab are represented. (B) Focus on relative expression of genes involved in the initiation of DNA replication after 24 h exposure to 20 μg ml−1 cetuximab (□), 5 μ
) and increased effect of oxaliplatin by more than 30% (▪) when combined with cetuximab are represented. (B) Focus on relative expression of genes involved in the initiation of DNA replication after 24 h exposure to 20 μg ml−1 cetuximab (□), 5 μ ), or a combination of both (▪). To normalise gene expression between treated and control samples, control genes of GAPDH and HPRT were used. Relative quantitation of mRNA expression of each gene was performed in triplicate.
), or a combination of both (▪). To normalise gene expression between treated and control samples, control genes of GAPDH and HPRT were used. Relative quantitation of mRNA expression of each gene was performed in triplicate.References
-
- Arnould S, Hennebelle I, Canal P, Bugat R, Guichard S (2003) Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur J Cancer 39: 112–119 - PubMed
-
- Balin-Gauthier D, Delord JP, Rochaix P, Mallard V, Thomas F, Hennebelle I, Bugat R, Canal P, Allal C (2006) In vivo and in vitro antitumor activity of oxaliplatin in combination with cetuximab in human colorectal tumor cell lines expressing different level of EGFR. Cancer Chemother Pharmacol 57: 709–718 - PubMed
-
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, Siena S, Bardelli A (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67(6): 2643–2648 - PubMed
-
- Blyumenberg G, Gorbacheva LB, Gorbunova VA, Kozachenko VP, Lankin VZ (1996) Factors of the ovarian cancer resistance to combined chemotherapy with platinum preparations. Bull Exp Biol Med 122(6): 1213–1216 - PubMed
-
- Carpenter G, Cohen S (1990) Epidermal growth factor. J Biol Chem 265: 7709–7712 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

