Protein-DNA chimeras for single molecule mechanical folding studies with the optical tweezers
- PMID: 18183383
- PMCID: PMC2862981
- DOI: 10.1007/s00249-007-0247-y
Protein-DNA chimeras for single molecule mechanical folding studies with the optical tweezers
Abstract
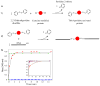
Here we report on a method that extends the study of the mechanical behavior of single proteins to the low force regime of optical tweezers. This experimental approach relies on the use of DNA handles to specifically attach the protein to polystyrene beads and minimize the non-specific interactions between the tethering surfaces. The handles can be attached to any exposed pair of cysteine residues. Handles of different lengths were employed to mechanically manipulate both monomeric and polymeric proteins. The low spring constant of the optical tweezers enabled us to monitor directly refolding events and fluctuations between different molecular structures in quasi-equilibrium conditions. This approach, which has already yielded important results on the refolding process of the protein RNase H (Cecconi et al. in Science 309: 2057-2060, 2005), appears robust and widely applicable to any protein engineered to contain a pair of reactive cysteine residues. It represents a new strategy to study protein folding at the single molecule level, and should be applicable to a range of problems requiring tethering of protein molecules.
Figures



References
-
- Berkemeier F, Schlierf M, Rief M. Mechanically controlled preparation of protein intermediates in single molecule experiments. Phys Status Solidi a-Appl Mater Sci. 2006;203:3492–3495.
-
- Brockwell DJ, Paci E, Zinober RC, Beddard GS, Olmsted PD, Smith DA, Perham RN, Radford SE. Pulling geometry defines the mechanical resistance of a beta-sheet protein. Nat Struct Biol. 2003;10:731–737. - PubMed
-
- Bustamante C, Chemla YR, Forde NR, Izhaky D. Mechanical processes in biochemistry. Annu Rev Biochem. 2004;73:705–748. - PubMed
-
- Bustamante C, Rivetti C, Keller DJ. Scanning force microscopy under aqueous solutions. Curr Opin Struct Biol. 1997;7:709–716. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

