Global amine and acid functional group modification of proteins
- PMID: 18184016
- PMCID: PMC2364710
- DOI: 10.1021/ac7019317
Global amine and acid functional group modification of proteins
Abstract
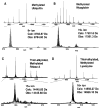
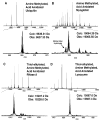
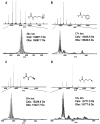
A sequential reaction methodology is employed for the complete derivatization of protein thiols, amines, and acids in high purity under denaturing conditions. Following standard thiol alkylation, protein amines are modified via reductive methylation with formaldehyde and pyridine-borane. Protein acids are subsequently amidated under buffered conditions in DMSO using the coupling reagent (7-azabenzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate. The generality of the approach is demonstrated with four proteins and with several amines yielding near-quantitative transformations as characterized by high-resolution Fourier transform mass spectrometry. The developed approach has numerous implications for protein characterization and general protein chemistry. Applications in mass spectrometry (MS) based proteomics of intact proteins (top-down MS) are explored, including the addition of stable isotopes for relative quantitation and protein identification through functional group counting. The methodology can be used for altering the physical and chemical properties of proteins, as demonstrated with amidation to modify protein isoelectric point and through derivatization with quaternary amines. Additionally, the chemistry has applications in the semisynthesis of monodisperse polymers based on protein scaffolds. We prepare proteins modified with azides and alkynes to enable further functionalization via copper(I)-catalyzed 1,3-dipolar Huisgen cycloaddition ("click") chemistry.
Figures

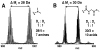
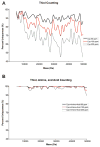
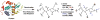
Similar articles
-
Complete chemical modification of amine and acid functional groups of peptides and small proteins.Methods Mol Biol. 2011;753:77-91. doi: 10.1007/978-1-61779-148-2_6. Methods Mol Biol. 2011. PMID: 21604117 Free PMC article.
-
Copper-catalyzed Huisgen and oxidative Huisgen coupling reactions controlled by polysiloxane-supported amines (AFPs) for the divergent synthesis of triazoles and bistriazoles.Chemistry. 2012 Oct 29;18(44):14094-9. doi: 10.1002/chem.201202472. Epub 2012 Sep 27. Chemistry. 2012. PMID: 23018981
-
Phosphoproteomics by mass spectrometry and classical protein chemistry approaches.Mass Spectrom Rev. 2005 Nov-Dec;24(6):828-46. doi: 10.1002/mas.20042. Mass Spectrom Rev. 2005. PMID: 15538747 Review.
-
Biomolecular assemblies combining two orthogonal copper-mediated ligations in a one-pot reaction.Chemistry. 2015 Apr 13;21(16):6022-6. doi: 10.1002/chem.201500293. Epub 2015 Mar 20. Chemistry. 2015. PMID: 25801963
-
Recent Advances in Recoverable Systems for the Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction (CuAAC).Molecules. 2016 Sep 5;21(9):1174. doi: 10.3390/molecules21091174. Molecules. 2016. PMID: 27607998 Free PMC article. Review.
Cited by
-
Complete chemical modification of amine and acid functional groups of peptides and small proteins.Methods Mol Biol. 2011;753:77-91. doi: 10.1007/978-1-61779-148-2_6. Methods Mol Biol. 2011. PMID: 21604117 Free PMC article.
-
Selective deletion of the internal lysine residue from the peptide sequence by collisional activation.J Am Soc Mass Spectrom. 2012 Nov;23(11):1967-80. doi: 10.1007/s13361-012-0456-1. Epub 2012 Aug 25. J Am Soc Mass Spectrom. 2012. PMID: 22923014
-
Ion-Ion Reactions with Fixed-Charge Modified Proteins to Produce Ions in a Single, Very High Charge State.Int J Mass Spectrom. 2008 Oct 1;276(2-3):136-143. doi: 10.1016/j.ijms.2008.07.029. Int J Mass Spectrom. 2008. PMID: 19802328 Free PMC article.
-
Methylofuran is a prosthetic group of the formyltransferase/hydrolase complex and shuttles one-carbon units between two active sites.Proc Natl Acad Sci U S A. 2019 Dec 17;116(51):25583-25590. doi: 10.1073/pnas.1911595116. Epub 2019 Nov 27. Proc Natl Acad Sci U S A. 2019. PMID: 31776258 Free PMC article.
-
Modifying the charge state distribution of proteins in electrospray ionization mass spectrometry by chemical derivatization.J Am Soc Mass Spectrom. 2009 Sep;20(9):1617-25. doi: 10.1016/j.jasms.2009.04.017. Epub 2009 May 4. J Am Soc Mass Spectrom. 2009. PMID: 19481956 Free PMC article.
References
-
- Julka S, Regnier F. J Proteome Res. 2004;3:350–363. - PubMed
-
- Leitner A, Lindner W. Proteomics. 2006;6:5418–5434. - PubMed
-
- Lundblad RL. Chemical Reagents for Protein Modification. 3. CRC Press; Boca Raton, FL: 2005.
-
- Zhao JY, Waldron KC, Miller J, Zhang JZ, Harke H, Dovicki NJ. J Chromatogr. 1992;608:239–242. - PubMed
-
- Means GE, Feeney RE. Anal Biochem. 1995;224:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

