Multisensory interplay reveals crossmodal influences on 'sensory-specific' brain regions, neural responses, and judgments
- PMID: 18184561
- PMCID: PMC2427054
- DOI: 10.1016/j.neuron.2007.12.013
Multisensory interplay reveals crossmodal influences on 'sensory-specific' brain regions, neural responses, and judgments
Abstract
Although much traditional sensory research has studied each sensory modality in isolation, there has been a recent explosion of interest in causal interplay between different senses. Various techniques have now identified numerous multisensory convergence zones in the brain. Some convergence may arise surprisingly close to low-level sensory-specific cortex, and some direct connections may exist even between primary sensory cortices. A variety of multisensory phenomena have now been reported in which sensory-specific brain responses and perceptual judgments concerning one sense can be affected by relations with other senses. We survey recent progress in this multisensory field, foregrounding human studies against the background of invasive animal work and highlighting possible underlying mechanisms. These include rapid feedforward integration, possible thalamic influences, and/or feedback from multisensory regions to sensory-specific brain areas. Multisensory interplay is more prevalent than classic modular approaches assumed, and new methods are now available to determine the underlying circuits.
Figures

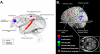
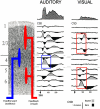


References
-
- Alais D., Burr D. The ventriloquist effect results from near-optimal bimodal integration. Curr. Biol. 2004;14:257–262. - PubMed
-
- Allman B.L., Meredith M.A. Multisensory processing in “unimodal” neurons: cross-modal subthreshold auditory effects in cat extrastriate visual cortex. J. Neurophysiol. 2007;98:545–549. - PubMed
-
- Amedi A., Jacobson G., Hendler T., Malach R., Zohary E. Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb. Cortex. 2002;12:1202–1212. - PubMed
-
- Arden G.B., Wolf J.E., Messiter C. Electrical activity in visual cortex associated with combined auditory and visual stimulation in temporal sequences known to be associated with a visual illusion. Vision Res. 2003;43:2469–2478. - PubMed
-
- Barbas H., Medalla M., Alade O., Suski J., Zikopoulos B., Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb. Cortex. 2005;15:1356–1370. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

