Cell-nonautonomous function of ceramidase in photoreceptor homeostasis
- PMID: 18184565
- PMCID: PMC2271043
- DOI: 10.1016/j.neuron.2007.10.041
Cell-nonautonomous function of ceramidase in photoreceptor homeostasis
Abstract
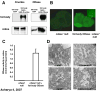
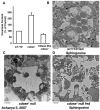
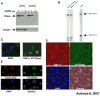
Neutral ceramidase, a key enzyme of sphingolipid metabolism, hydrolyzes ceramide to sphingosine. These sphingolipids are critical structural components of cell membranes and act as second messengers in diverse signal transduction cascades. Here, we have isolated and characterized functional null mutants of Drosophila ceramidase. We show that secreted ceramidase functions in a cell-nonautonomous manner to maintain photoreceptor homeostasis. In the absence of ceramidase, photoreceptors degenerate in a light-dependent manner, are defective in normal endocytic turnover of rhodopsin, and do not respond to light stimulus. Consistent with a cell-nonautonomous function, overexpression of ceramidase in tissues distant from photoreceptors suppresses photoreceptor degeneration in an arrestin mutant and facilitates membrane turnover in a rhodopsin null mutant. Furthermore, our results show that secreted ceramidase is internalized and localizes to endosomes. Our findings establish a role for a secreted sphingolipid enzyme in the regulation of photoreceptor structure and function.
Figures





References
-
- Acharya U, Patel S, Koundakjian E, Nagashima K, Han X, Acharya JK. Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science. 2003;299:1740–1743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

