Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses
- PMID: 18184568
- PMCID: PMC2390917
- DOI: 10.1016/j.neuron.2007.11.024
Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses
Abstract
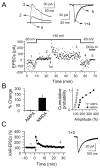
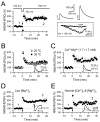
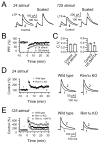
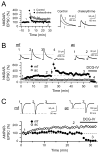
The mossy fiber to CA3 pyramidal cell synapse (mf-CA3) provides a major source of excitation to the hippocampus. Thus far, these glutamatergic synapses are well recognized for showing a presynaptic, NMDA receptor-independent form of LTP that is expressed as a long-lasting increase of transmitter release. Here, we show that in addition to this "classical" LTP, mf-CA3 synapses can undergo a form of LTP characterized by a selective enhancement of NMDA receptor-mediated transmission. This potentiation requires coactivation of NMDA and mGlu5 receptors and a postsynaptic calcium rise. Unlike classical LTP, expression of this mossy fiber LTP is due to a PKC-dependent recruitment of NMDA receptors specifically to the mf-CA3 synapse via a SNARE-dependent process. Having two mechanistically different forms of LTP may allow mf-CA3 synapses to respond with more flexibility to the changing demands of the hippocampal network.
Figures




Comment in
-
The two sides of hippocampal mossy fiber plasticity.Neuron. 2008 Jan 10;57(1):5-7. doi: 10.1016/j.neuron.2007.12.015. Neuron. 2008. PMID: 18184559
Similar articles
-
Bidirectional Hebbian plasticity at hippocampal mossy fiber synapses on CA3 interneurons.J Neurosci. 2008 Dec 24;28(52):14042-55. doi: 10.1523/JNEUROSCI.4848-08.2008. J Neurosci. 2008. PMID: 19109487 Free PMC article.
-
Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses.Eur J Neurosci. 2004 Jul;20(1):101-10. doi: 10.1111/j.1460-9568.2004.03461.x. Eur J Neurosci. 2004. PMID: 15245483
-
Photolysis of postsynaptic caged Ca2+ can potentiate and depress mossy fiber synaptic responses in rat hippocampal CA3 pyramidal neurons.J Neurophysiol. 2004 Apr;91(4):1596-607. doi: 10.1152/jn.01073.2003. Epub 2003 Nov 26. J Neurophysiol. 2004. PMID: 14645386 Free PMC article.
-
A peculiar form of potentiation in mossy fiber synapses.Epilepsy Res Suppl. 1992;7:151-7. Epilepsy Res Suppl. 1992. PMID: 1334660 Review.
-
Multiple forms of long-term synaptic plasticity at hippocampal mossy fiber synapses on interneurons.Neuropharmacology. 2011 Apr;60(5):740-7. doi: 10.1016/j.neuropharm.2010.11.008. Epub 2010 Nov 18. Neuropharmacology. 2011. PMID: 21093459 Free PMC article. Review.
Cited by
-
CaMKII-dependent phosphorylation of GluK5 mediates plasticity of kainate receptors.EMBO J. 2013 Feb 20;32(4):496-510. doi: 10.1038/emboj.2012.334. Epub 2013 Jan 4. EMBO J. 2013. PMID: 23288040 Free PMC article.
-
The Role of mGlu Receptors in Hippocampal Plasticity Deficits in Neurological and Psychiatric Disorders: Implications for Allosteric Modulators as Novel Therapeutic Strategies.Curr Neuropharmacol. 2016;14(5):455-73. doi: 10.2174/1570159x13666150421003225. Curr Neuropharmacol. 2016. PMID: 27296640 Free PMC article. Review.
-
Therapeutic potential of metabotropic glutamate receptor modulators.Curr Neuropharmacol. 2012 Mar;10(1):12-48. doi: 10.2174/157015912799362805. Curr Neuropharmacol. 2012. PMID: 22942876 Free PMC article.
-
cAMP-Dependent Synaptic Plasticity at the Hippocampal Mossy Fiber Terminal.Front Synaptic Neurosci. 2022 Apr 4;14:861215. doi: 10.3389/fnsyn.2022.861215. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35444523 Free PMC article. Review.
-
Presynaptic long-term plasticity.Front Synaptic Neurosci. 2013 Oct 17;5:8. doi: 10.3389/fnsyn.2013.00008. Front Synaptic Neurosci. 2013. PMID: 24146648 Free PMC article. Review.
References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Aniksztejn L, Ben-Ari Y. Expression of LTP by AMPA and/or NMDA receptors is determined by the extent of NMDA receptors activation during the tetanus. J Neurophysiol. 1995;74:2349–2357. - PubMed
-
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. - PubMed
-
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. - PubMed
-
- Bayazitov I, Kleschevnikov A. Afferent high strength tetanizations favour potentiation of the NMDA vs. AMPA receptor-mediated component of field EPSP in CA1 hippocampal slices of rats. Brain Res. 2000;866:188–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

