Hydrodynamic aspects of fish olfaction
- PMID: 18184629
- PMCID: PMC2408354
- DOI: 10.1098/rsif.2007.1281
Hydrodynamic aspects of fish olfaction
Abstract
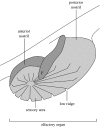
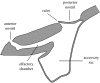
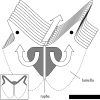
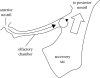
Flow into and around the olfactory chamber of a fish determines how odorant from the fish's immediate environment is transported to the sensory surface (olfactory epithelium) lining the chamber. Diffusion times in water are long, even over comparatively short distances (millimetres). Therefore, transport from the external environment to the olfactory epithelium must be controlled by processes that rely on convection (i.e. the bulk flow of fluid). These include the beating of cilia lining the olfactory chamber and the relatively inexpensive pumping action of accessory sacs. Flow through the chamber may also be induced by an external flow. Flow over the olfactory epithelium appears to be laminar. Odorant transfer to the olfactory epithelium may be facilitated in several ways: if the olfactory organs are mounted on stalks that penetrate the boundary layer; by the steep velocity gradients generated by beating cilia; by devices that deflect flow into the olfactory chamber; by parallel arrays of olfactory lamellae; by mechanical agitation of the chamber (or olfactory stalks); and by vortices. Overall, however, our knowledge of the hydrodynamics of fish olfaction is far from complete. Several areas of future research are outlined.
Figures







Similar articles
-
A computational study of the hydrodynamics in the nasal region of a hammerhead shark (Sphyrna tudes): implications for olfaction.PLoS One. 2013;8(3):e59783. doi: 10.1371/journal.pone.0059783. Epub 2013 Mar 29. PLoS One. 2013. PMID: 23555780 Free PMC article.
-
Three-dimensional structure of the nasal passageway of a hagfish and its implications for olfaction.Anat Rec (Hoboken). 2011 Jun;294(6):1045-56. doi: 10.1002/ar.21382. Epub 2011 Apr 28. Anat Rec (Hoboken). 2011. PMID: 21538925
-
Motion-driven flow in an unusual piscine nasal region.Zoology (Jena). 2016 Dec;119(6):500-510. doi: 10.1016/j.zool.2016.06.008. Epub 2016 Jul 6. Zoology (Jena). 2016. PMID: 27449820
-
Olfaction in fish.Prog Neurobiol. 1975;5(4):271-335. doi: 10.1016/0301-0082(75)90014-3. Prog Neurobiol. 1975. PMID: 830087 Review.
-
Olfactory toxicity in fishes.Aquat Toxicol. 2010 Jan 21;96(1):2-26. doi: 10.1016/j.aquatox.2009.09.019. Epub 2009 Oct 30. Aquat Toxicol. 2010. PMID: 19931199 Review.
Cited by
-
How the evolution of air breathing shaped hippocampal function.Philos Trans R Soc Lond B Biol Sci. 2022 Feb 14;377(1844):20200532. doi: 10.1098/rstb.2020.0532. Epub 2021 Dec 27. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 34957846 Free PMC article.
-
Gross morphology, histology, and ultrastructure of the olfactory rosette of a critically endangered indicator species, the Delta Smelt, Hypomesus transpacificus.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2021 Sep;207(5):597-616. doi: 10.1007/s00359-021-01500-7. Epub 2021 Jun 22. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2021. PMID: 34156533 Free PMC article.
-
Lacustrine speciation associated with chromosomal inversion in a lineage of riverine fishes.Evolution. 2023 Jun 29;77(7):1505-1521. doi: 10.1093/evolut/qpad067. Evolution. 2023. PMID: 37094800 Free PMC article.
-
Ontogenetic shifts in olfactory rosette morphology of the sockeye salmon, Oncorhynchus nerka.J Morphol. 2023 Jan;284(1):e21539. doi: 10.1002/jmor.21539. J Morphol. 2023. PMID: 36433755 Free PMC article.
-
An optofluidic platform for interrogating chemosensory behavior and brainwide neural representation in larval zebrafish.Nat Commun. 2023 Jan 14;14(1):227. doi: 10.1038/s41467-023-35836-2. Nat Commun. 2023. PMID: 36641479 Free PMC article.
References
-
- Alexander R.McN. Structure and function in the catfish. J. Zool. 1965;148:88–152.
-
- Anderson E.J, McGillis W.R, Grosenbaugh M.A. The boundary layer of swimming fish. J. Exp. Biol. 2001;204:81–102. - PubMed
-
- Anon. Fish images provide net benefit to researchers. Nature. 2006;440:396–397. doi: 10.1038/440396a. - DOI
-
- Atema J. Distribution of chemical stimuli. In: Atema J, Fay R.R, Popper A.N, Tavolga W.N, editors. Sensory biology of aquatic animals. Springer; New York, NY: 1988. pp. 29–56.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

