Sialic acid is a cellular receptor for coxsackievirus A24 variant, an emerging virus with pandemic potential
- PMID: 18184708
- PMCID: PMC2259016
- DOI: 10.1128/JVI.02470-07
Sialic acid is a cellular receptor for coxsackievirus A24 variant, an emerging virus with pandemic potential
Erratum in
- J Virol. 2008 May;82(10):5115
Abstract
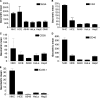
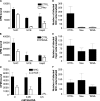
Binding to target cell receptors is a critical step in the virus life cycle. Coxsackievirus A24 variant (CVA24v) has pandemic potential and is a major cause of acute hemorrhagic conjunctivitis, but its cellular receptor has hitherto been unknown. Here we show that CVA24v fails to bind to and infect CHO cells defective in sialic acid expression. Binding of CVA24v to and infection of corneal epithelial cells are efficiently inhibited by treating cells with a sialic acid-cleaving enzyme or sialic acid-binding lectins and by treatment of the virus with soluble, multivalent sialic acid. Protease treatment of cells efficiently inhibited virus binding, suggesting that the receptor is a sialylated glycoprotein. Like enterovirus type 70 and influenza A virus, CVA24v can cause pandemics. Remarkably, all three viruses use the same receptor. Since several unrelated viruses with tropism for the eye use this receptor, sialic acid-based antiviral drugs that prevent virus entry may be useful for topical treatment of such infections.
Figures




References
-
- Alonso-Echanove, J., Y. García-Guadalupe, P. Rullán, M. A. Pallansch, F. Alvarado-Ramy, and B. Cauthen. 2004. Acute hemorrhagic conjunctivitis outbreak caused by coxsackievirus A24—Puerto Rico, 2003. Morb. Mortal. Wkly. Rep. 53632-634. - PubMed
-
- Aoki, K., and H. Sawada. 1992. Long-term observation of neutralization antibody after enterovirus 70 infection. Jpn. J. Ophthalmol. 36465-468. - PubMed
-
- Araki-Sasaki, K., K. Y. Ohasi, T. Sasabe, K. Hayashi, H. Watanabe, Y. Tano, and H. Handa. 1995. An SV-40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. 36614-621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

