Geometric mismatches within the concentric layers of rotavirus particles: a potential regulatory switch of viral particle transcription activity
- PMID: 18184711
- PMCID: PMC2258973
- DOI: 10.1128/JVI.02268-07
Geometric mismatches within the concentric layers of rotavirus particles: a potential regulatory switch of viral particle transcription activity
Abstract
Rotaviruses are prototypical double-stranded RNA viruses whose triple-layered icosahedral capsid constitutes transcriptional machinery activated by the release of the external layer. To understand the molecular basis of this activation, we studied the structural interplay between the three capsid layers by electron cryo-microscopy and digital image processing. Two viral particles and four virus-like particles containing various combinations of inner (VP2)-, middle (VP6)-, and outer (VP7)-layer proteins were studied. We observed that the absence of the VP2 layer increases the particle diameter and changes the type of quasi-equivalent icosahedral symmetry, as described by the shift in triangulation number (T) of the VP6 layer (from T = 13 to T = 19 or more). By fitting X-ray models of VP6 into each reconstruction, we determined the quasi-atomic structures of the middle layers. These models showed that the VP6 lattices, i.e., curvature and trimer contacts, are characteristic of the particle composition. The different functional states of VP6 thus appear as being characterized by trimers having similar conformations but establishing different intertrimeric contacts. Remarkably, the external protein VP7 reorients the VP6 trimers located around the fivefold axes of the icosahedral capsid, thereby shrinking the channel through which mRNA exits the transcribing rotavirus particle. We conclude that the constraints arising from the different geometries imposed by the external and internal layers of the rotavirus capsid constitute a potential switch regulating the transcription activity of the viral particles.
Figures

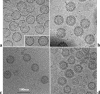



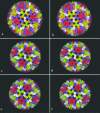
Similar articles
-
Identification of rotavirus VP6 residues located at the interface with VP2 that are essential for capsid assembly and transcriptase activity.J Virol. 2002 Aug;76(15):7822-31. doi: 10.1128/jvi.76.15.7822-7831.2002. J Virol. 2002. PMID: 12097594 Free PMC article.
-
Production of hybrid double- or triple-layered virus-like particles of group A and C rotaviruses using a baculovirus expression system.Virology. 2002 Oct 10;302(1):1-8. doi: 10.1006/viro.2002.1610. Virology. 2002. PMID: 12429511
-
Projection structure of VP6, the rotavirus inner capsid protein, and comparison with bluetongue VP7.J Mol Biol. 1997 Sep 26;272(3):362-8. doi: 10.1006/jmbi.1997.1179. J Mol Biol. 1997. PMID: 9325096
-
Emerging themes in rotavirus cell entry, genome organization, transcription and replication.Virus Res. 2004 Apr;101(1):67-81. doi: 10.1016/j.virusres.2003.12.007. Virus Res. 2004. PMID: 15010218 Review.
-
Rotavirus Replication: Gaps of Knowledge on Virus Entry and Morphogenesis.Tohoku J Exp Med. 2019 Aug;248(4):285-296. doi: 10.1620/tjem.248.285. Tohoku J Exp Med. 2019. PMID: 31447474 Review.
Cited by
-
Structural insights into the coupling of virion assembly and rotavirus replication.Nat Rev Microbiol. 2012 Jan 23;10(3):165-77. doi: 10.1038/nrmicro2673. Nat Rev Microbiol. 2012. PMID: 22266782 Free PMC article. Review.
-
Human rotavirus VP6-specific antibodies mediate intracellular neutralization by binding to a quaternary structure in the transcriptional pore.PLoS One. 2013 May 9;8(5):e61101. doi: 10.1371/journal.pone.0061101. Print 2013. PLoS One. 2013. PMID: 23671563 Free PMC article.
-
Periodic table of virus capsids: implications for natural selection and design.PLoS One. 2010 Mar 4;5(3):e9423. doi: 10.1371/journal.pone.0009423. PLoS One. 2010. PMID: 20209096 Free PMC article.
-
Mechanism of intraparticle synthesis of the rotavirus double-stranded RNA genome.J Biol Chem. 2010 Jun 11;285(24):18123-8. doi: 10.1074/jbc.R110.117671. Epub 2010 Mar 29. J Biol Chem. 2010. PMID: 20351108 Free PMC article.
-
Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM.Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10644-8. doi: 10.1073/pnas.0904024106. Epub 2009 Jun 1. Proc Natl Acad Sci U S A. 2009. PMID: 19487668 Free PMC article.
References
-
- Adrian, M., J. Dubochet, J. Lepault, and A. W. McDowall. 1984. Cryo-electron microscopy of viruses. Nature 30832-36. - PubMed
-
- Benureau, Y., J. C. Huet, A. Charpilienne, D. Poncet, and J. Cohen. 2005. Trypsin is associated with the rotavirus capsid and is activated by solubilization of outer capsid proteins. J. Gen. Virol. 863143-3151. - PubMed
-
- Caspar, D. L. D., and A. Klug. 1962. Physical principles in the construction of regular viruses. Cold Spring Harbor Symp. Quant. Biol. 271-24. - PubMed
-
- Charpilienne, A., M. Nejmeddine, M. Berois, N. Parez, E. Neumann, E. Hewat, G. Trugnan, and J. Cohen. 2001. Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J. Biol. Chem. 27629361-29367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

