Differential CD4+ versus CD8+ T-cell responses elicited by different poxvirus-based human immunodeficiency virus type 1 vaccine candidates provide comparable efficacies in primates
- PMID: 18184713
- PMCID: PMC2258966
- DOI: 10.1128/JVI.02216-07
Differential CD4+ versus CD8+ T-cell responses elicited by different poxvirus-based human immunodeficiency virus type 1 vaccine candidates provide comparable efficacies in primates
Abstract
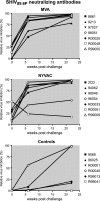
Poxvirus vectors have proven to be highly effective for boosting immune responses in diverse vaccine settings. Recent reports reveal marked differences in the gene expression of human dendritic cells infected with two leading poxvirus-based human immunodeficiency virus (HIV) vaccine candidates, New York vaccinia virus (NYVAC) and modified vaccinia virus Ankara (MVA). To understand how complex genomic changes in these two vaccine vectors translate into antigen-specific systemic immune responses, we undertook a head-to-head vaccine immunogenicity and efficacy study in the pathogenic HIV type 1 (HIV-1) model of AIDS in Indian rhesus macaques. Differences in the immune responses in outbred animals were not distinguished by enzyme-linked immunospot assays, but differences were distinguished by multiparameter fluorescence-activated cell sorter analysis, revealing a difference between the number of animals with both CD4(+) and CD8(+) T-cell responses to vaccine inserts (MVA) and those that elicit a dominant CD4(+) T-cell response (NYVAC). Remarkably, vector-induced differences in CD4(+)/CD8(+) T-cell immune responses persisted for more than a year after challenge and even accompanied antigenic modulation throughout the control of chronic infection. Importantly, strong preexposure HIV-1/simian immunodeficiency virus-specific CD4(+) T-cell responses did not prove deleterious with respect to accelerated disease progression. In contrast, in this setting, animals with strong vaccine-induced polyfunctional CD4(+) T-cell responses showed efficacies similar to those with stronger CD8(+) T-cell responses.
Figures







References
-
- Albert, M. L. 2004. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat. Rev. Immunol. 4223-231. - PubMed
-
- Amara, R. R., C. Ibegbu, F. Villinger, D. C. Montefiori, S. Sharma, P. Nigam, Y. Xu, H. M. McClure, and H. L. Robinson. 2005. Studies using a viral challenge and CD8 T cell depletions on the roles of cellular and humoral immunity in the control of an SHIV-89.6P challenge in DNA/MVA-vaccinated macaques. Virology 343246-255. - PubMed
-
- Amara, R. R., K. Patel, G. Niedziela, P. Nigam, S. Sharma, S. I. Staprans, D. C. Montefiori, L. Chenareddi, J. G. Herndon, H. L. Robinson, H. M. McClure, and F. J. Novembre. 2005. A combination DNA and attenuated simian immunodeficiency virus vaccine strategy provides enhanced protection from simian/human immunodeficiency virus-induced disease. J. Virol. 7915356-15367. - PMC - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. - PubMed
-
- Anel, A., A. Bosque, J. Naval, A. Pineiro, L. Larrad, M. A. Alava, and M. J. Martinez-Lorenzo. 2007. Apo2L/TRAIL and immune regulation. Front. Biosci. 122074-2084. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

