The DNA deaminase activity of human APOBEC3G is required for Ty1, MusD, and human immunodeficiency virus type 1 restriction
- PMID: 18184715
- PMCID: PMC2259018
- DOI: 10.1128/JVI.02391-07
The DNA deaminase activity of human APOBEC3G is required for Ty1, MusD, and human immunodeficiency virus type 1 restriction
Abstract
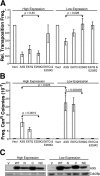
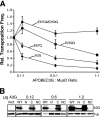
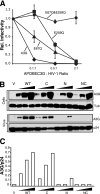
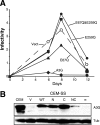
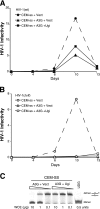
Human APOBEC3G and several other APOBEC3 proteins have been shown to inhibit the replication of a variety of retrotransposons and retroviruses. All of these enzymes can deaminate cytosines within single-strand DNA, but the overall importance of this conserved activity in retroelement restriction has been questioned by reports of deaminase-independent mechanisms. Here, three distinct retroelements, a yeast retrotransposon, Ty1, a murine endogenous retrovirus, MusD, and a lentivirus, human immunodeficiency virus type 1 (HIV-1), were used to evaluate the relative contributions of deaminase-dependent and -independent mechanisms. Although human APOBEC3G can restrict the replication of all three of these retroelements, APOBEC3G lacking the catalytic glutamate (E259Q) was clearly defective. This phenotype was particularly clear in experiments with low levels of APOBEC3G expression. In contrast, purposeful overexpression of APOBEC3G-E259Q was able to cause modest to severe reductions in the replication of Ty1, MusD, and HIV-1(DeltaVif). The importance of these observations was highlighted by data showing that CEM-SS T-cell lines expressing near-physiologic levels of APOBEC3G-E259Q failed to inhibit the replication of HIV-1(DeltaVif), whereas similar levels of wild-type APOBEC3G fully suppressed virus infectivity. Despite the requirement for DNA deamination, uracil DNA glycosylase did not modulate APOBEC3G-dependent restriction of Ty1 or HIV-1(DeltaVif), further supporting prior studies indicating that the major uracil excision repair system of cells is not involved. In conclusion, the absolute requirement for the catalytic glutamate of APOBEC3G in Ty1, MusD, and HIV-1 restriction strongly indicates that DNA cytosine deamination is an essential part of the mechanism.
Figures


References
-
- Bieniasz, P. D. 2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 51109-1115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

