SEUSS and AINTEGUMENTA mediate patterning and ovule initiation during gynoecium medial domain development
- PMID: 18184731
- PMCID: PMC2259068
- DOI: 10.1104/pp.107.114751
SEUSS and AINTEGUMENTA mediate patterning and ovule initiation during gynoecium medial domain development
Abstract
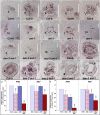
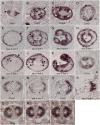
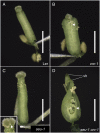
The Arabidopsis (Arabidopsis thaliana) gynoecium, the female floral reproductive structure, requires the action of genes that specify positional identities during its development to generate an organ competent for seed development and dispersal. Early in gynoecial development, patterning events divide the primordium into distinct domains that will give rise to specific tissues and organs. The medial domain of the gynoecium gives rise to the ovules, and several other structures critical for reproductive competence. Here we report a synergistic genetic interaction between seuss and aintegumenta mutants resulting in a complete loss of ovule initiation and a reduction of the structures derived from the medial domain. We show that patterning events are disrupted early in the development of the seuss aintegumenta gynoecia and we identify PHABULOSA (PHB), REVOLUTA, and CRABS CLAW (CRC) as potential downstream targets of SEUSS (SEU) and AINTEGUMENTA (ANT) regulation. Our genetic data suggest that SEU additionally functions in pathways that are partially redundant and parallel to PHB, CRC, and ANT. Thus, SEU and ANT are part of a complex and robust molecular system that coordinates patterning cues and cellular proliferation along the three positional axes of the developing gynoecium.
Figures





References
-
- Alvarez J, Smyth DR (1999) CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development 126 2377–2386 - PubMed
-
- Alvarez J, Smyth DR (2002) CRABS CLAW and SPATULA genes regulate growth and pattern formation during gynoecium development in Arabidopsis thaliana. Int J Plant Sci 163 17–41
-
- Balanza V, Navarrete M, Trigueros M, Ferrandiz C (2006) Patterning the female side of Arabidopsis: the importance of hormones. J Exp Bot 57 3457–3469 - PubMed
-
- Berleth T, Jurgens G (1993) The role of monopteros in organizing the basal body region of the Arabidopsis embryo. Development 118 575–587
-
- Bowman JL, Baum SF, Eshed Y, Putterill J, Alvarez J (1999) Molecular genetics of gynoecium development in Arabidopsis. Curr Top Dev Biol 45 155–205 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

