Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation
- PMID: 18184788
- PMCID: PMC3057190
- DOI: 10.1523/JNEUROSCI.2735-07.2008
Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation
Abstract
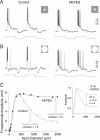
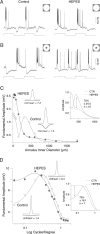
Negative feedback from horizontal cells to cone photoreceptors is regarded as the critical pathway for the formation of the antagonistic surround of retinal neurons, yet the mechanism by which horizontal cells accomplish negative feedback has been difficult to determine. Recent evidence suggests that feedback uses a novel, non-GABAergic pathway that directly modulates the calcium current in cones. In non-mammalian vertebrates, enrichment of retinal pH buffering capacity attenuates horizontal cell feedback, supporting one model in which feedback occurs by horizontal cell modulation of the extracellular pH in the cone synaptic cleft. Here we test the effect of exogenous pH buffering on the response dynamics of H1 horizontal cells and the center-surround receptive field structure of parasol ganglion cells in the macaque monkey retina. Enrichment of the extracellular buffering capacity with HEPES selectively attenuates surround antagonism in parasol ganglion cells. The H1 horizontal cell light response includes a slow, depolarizing component that is attributed to negative feedback to cones. This part of the response is attenuated by HEPES and other pH buffers in a dose-dependent manner that is correlated with predicted buffering capacity. The selective effects of pH buffering on the parasol cell surround and H1 cell light response suggests that, in primate retina, horizontal cell feedback to cones is mediated via a pH-dependent mechanism and is a major determinant of the ganglion cell receptive field surround.
Figures





Similar articles
-
Sources of protons and a role for bicarbonate in inhibitory feedback from horizontal cells to cones in Ambystoma tigrinum retina.J Physiol. 2016 Nov 15;594(22):6661-6677. doi: 10.1113/JP272533. Epub 2016 Jul 21. J Physiol. 2016. PMID: 27345444 Free PMC article.
-
Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina.J Neurosci. 2009 Jul 1;29(26):8372-87. doi: 10.1523/JNEUROSCI.1218-09.2009. J Neurosci. 2009. PMID: 19571128 Free PMC article.
-
The classical receptive field surround of primate parasol ganglion cells is mediated primarily by a non-GABAergic pathway.J Neurosci. 2004 Apr 14;24(15):3736-45. doi: 10.1523/JNEUROSCI.5252-03.2004. J Neurosci. 2004. PMID: 15084653 Free PMC article.
-
Distinct synaptic mechanisms create parallel S-ON and S-OFF color opponent pathways in the primate retina.Vis Neurosci. 2014 Mar;31(2):139-51. doi: 10.1017/S0952523813000230. Epub 2013 Jul 29. Vis Neurosci. 2014. PMID: 23895762 Free PMC article. Review.
-
Horizontal Cell Feedback to Cone Photoreceptors in Mammalian Retina: Novel Insights From the GABA-pH Hybrid Model.Front Cell Neurosci. 2020 Nov 4;14:595064. doi: 10.3389/fncel.2020.595064. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33328894 Free PMC article. Review.
Cited by
-
Differential encoding of spatial information among retinal on cone bipolar cells.J Neurophysiol. 2015 Sep;114(3):1757-72. doi: 10.1152/jn.00287.2015. Epub 2015 Jul 22. J Neurophysiol. 2015. PMID: 26203104 Free PMC article.
-
Tandem pore domain acid-sensitive K channel 3 (TASK-3) regulates visual sensitivity in healthy and aging retina.Sci Adv. 2022 Sep 9;8(36):eabn8785. doi: 10.1126/sciadv.abn8785. Epub 2022 Sep 7. Sci Adv. 2022. PMID: 36070380 Free PMC article.
-
Diversity of Ganglion Cell Responses to Saccade-Like Image Shifts in the Primate Retina.J Neurosci. 2023 Jul 19;43(29):5319-5339. doi: 10.1523/JNEUROSCI.1561-22.2023. Epub 2023 Jun 20. J Neurosci. 2023. PMID: 37339877 Free PMC article.
-
Lateral interactions in the outer retina.Prog Retin Eye Res. 2012 Sep;31(5):407-41. doi: 10.1016/j.preteyeres.2012.04.003. Epub 2012 May 3. Prog Retin Eye Res. 2012. PMID: 22580106 Free PMC article. Review.
-
Extracellular pH dynamics of retinal horizontal cells examined using electrochemical and fluorometric methods.J Neurophysiol. 2012 Feb;107(3):868-79. doi: 10.1152/jn.00878.2011. Epub 2011 Nov 16. J Neurophysiol. 2012. PMID: 22090459 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
