Wild-type but not FAD mutant presenilin-1 prevents neuronal degeneration by promoting phosphatidylinositol 3-kinase neuroprotective signaling
- PMID: 18184791
- PMCID: PMC6670519
- DOI: 10.1523/JNEUROSCI.4067-07.2008
Wild-type but not FAD mutant presenilin-1 prevents neuronal degeneration by promoting phosphatidylinositol 3-kinase neuroprotective signaling
Abstract
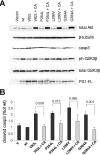
The role of presenilin-1 (PS1) in neuronal phosphatidylinositol 3-kinase (PI3K)/Akt signaling was investigated in primary neuronal cultures from wild-type (WT) and PS1 null (PS1-/-) embryonic mouse brains. Here we show that in PS1-/- cultures, the onset of neuronal maturation coincides with a decrease in the PI3K-dependent phosphorylation-activation of Akt and phosphorylation-inactivation of glycogen synthase kinase-3 (GSK-3). Mature PS1-/- neurons show increased activation of apoptotic caspase-3 and progressive degeneration preceded by dendritic retraction. Expression of exogenous WT PS1 or constitutively active Akt in PS1-/- neurons stimulates PI3K signaling and suppresses both caspase-3 activity and dendrite retraction. The survival effects of PS1 are sensitive to inhibitors of PI3K kinase but insensitive to gamma-secretase inhibitors. Familial Alzheimer disease (FAD) mutations suppress the ability of PS1 to promote PI3K/AKT signaling, prevent phosphorylation/inactivation of GSK-3 and promote activation of caspase-3. These mutation effects are reversed upon coexpression of constitutively active Akt. Together, our data indicate that the neuroprotective role of PS1 depends on its ability to activate the PI3K/Akt signaling pathway and that PS1 FAD mutations increase GSK-3 activity and promote neuronal apoptosis by inhibiting the function of PS1 in this pathway. These observations suggest that stimulation of PI3K/Akt signaling may be beneficial to FAD patients.
Figures




References
-
- Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004;89:1313–1317. - PubMed
-
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. - PubMed
-
- Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3β (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
