Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats
- PMID: 18184792
- PMCID: PMC6670515
- DOI: 10.1523/JNEUROSCI.1585-07.2008
Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats
Abstract
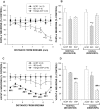
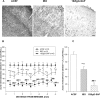
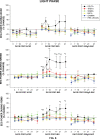
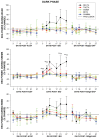
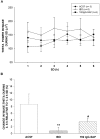
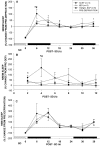
The basal forebrain (BF) is known for its role in cortical and behavioral activation, and has been postulated to have a role in compensatory mechanisms after sleep loss. However, specific neuronal phenotypes responsible for these roles are unclear. We investigated the effects of ibotenate (IBO) and 192IgG-saporin (SAP) lesions of the caudal BF on spontaneous sleep-waking and electroencephalogram (EEG), and recovery sleep and EEG after 6 h of sleep deprivation (SD). Relative to artificial CSF (ACSF) controls, IBO injections decreased parvalbumin and cholinergic neurons in the caudal BF by 43 and 21%, respectively, and cortical acetylcholinesterase staining by 41%. SAP injections nonsignificantly decreased parvalbumin neurons by 11%, but significantly decreased cholinergic neurons by 69% and cortical acetylcholinesterase by 84%. IBO lesions had no effect on sleep-wake states but increased baseline delta power in all states [up to 62% increase during non-rapid eye movement (NREM) sleep]. SAP lesions transiently increased NREM sleep by 13%, predominantly during the dark phase, with no effect on EEG. During the first 12 h after SD, animals with IBO and SAP lesions showed lesser rebound NREM sleep (32 and 77% less, respectively) and delta power (78 and 53% less) relative to ACSF controls. These results suggest that noncholinergic BF neurons promote cortical activation by inhibiting delta waves, whereas cholinergic BF neurons play a nonexclusive role in promoting wake. Intriguingly, these results also suggest that both types of BF neurons play important roles, probably through different mechanisms, in increased NREM sleep and EEG delta power after sleep loss.
Figures






References
-
- Arrigoni E, Chamberlin NL, Saper CB, McCarley RW. Adenosine inhibits basal forebrain cholinergic and noncholinergic neurons in vitro. Neuroscience. 2006;140:403–413. - PubMed
-
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. - PubMed
-
- Bassant MH, Apartis E, Jazat-Poindessous FR, Wiley RG, Lamour YA. Selective immunolesion of the basal forebrain cholinergic neurons: effects on hippocampal activity during sleep and wakefulness in the rat. Neurodegeneration. 1995;4:61–70. - PubMed
-
- Berntson GG, Shafi R, Sarter M. Specific contributions of the basal forebrain corticopetal cholinergic system to electroencephalographic activity and sleep/waking behaviour. Eur J Neurosci. 2002;16:2453–2461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
