Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide
- PMID: 18184806
- PMCID: PMC2206563
- DOI: 10.1073/pnas.0709191105
Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide
Abstract
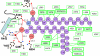
In mammalian cells, ceramide is synthesized in the endoplasmic reticulum and transferred to the Golgi apparatus for conversion to sphingomyelin. Ceramide transport occurs in a nonvesicular manner and is mediated by CERT, a cytosolic 68-kDa protein with a C-terminal steroidogenic acute regulatory protein-related lipid transfer (START) domain. The CERT START domain efficiently transfers natural D-erythro-C16-ceramide, but not lipids with longer (C20) amide-acyl chains. The molecular mechanisms of ceramide specificity, both stereo-specific recognition and length limit, are not well understood. Here we report the crystal structures of the CERT START domain in its apo-form and in complex with ceramides having different acyl chain lengths. In these complex structures, one ceramide molecule is buried in a long amphiphilic cavity. At the far end of the cavity, the amide and hydroxyl groups of ceramide form a hydrogen bond network with specific amino acid residues that play key roles in stereo-specific ceramide recognition. At the head of the ceramide molecule, there is no extra space to accommodate additional bulky groups. The two aliphatic chains of ceramide are surrounded by the hydrophobic wall of the cavity, whose size and shape dictate the length limit for cognate ceramides. Furthermore, local high-crystallographic B-factors suggest that the alpha-3 and the Omega1 loop might work as a gate to incorporate the ceramide into the cavity. Thus, the structures demonstrate the structural basis for the mechanism by which CERT can distinguish ceramide from other lipid types yet still recognize multiple species of ceramides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



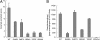

Similar articles
-
Crystal structures of the CERT START domain with inhibitors provide insights into the mechanism of ceramide transfer.J Mol Biol. 2010 Feb 19;396(2):245-51. doi: 10.1016/j.jmb.2009.12.029. Epub 2009 Dec 28. J Mol Biol. 2010. PMID: 20036255
-
CERT mediates intermembrane transfer of various molecular species of ceramides.J Biol Chem. 2005 Feb 25;280(8):6488-95. doi: 10.1074/jbc.M409290200. Epub 2004 Dec 13. J Biol Chem. 2005. PMID: 15596449
-
The intermembrane ceramide transport catalyzed by CERT is sensitive to the lipid environment.Biochim Biophys Acta. 2011 Jan;1808(1):229-35. doi: 10.1016/j.bbamem.2010.09.011. Epub 2010 Sep 25. Biochim Biophys Acta. 2011. PMID: 20875392
-
CERT and intracellular trafficking of ceramide.Biochim Biophys Acta. 2007 Jun;1771(6):644-53. doi: 10.1016/j.bbalip.2007.01.009. Epub 2007 Jan 23. Biochim Biophys Acta. 2007. PMID: 17314061 Review.
-
CERT-mediated trafficking of ceramide.Biochim Biophys Acta. 2009 Jul;1791(7):684-91. doi: 10.1016/j.bbalip.2009.01.006. Epub 2009 Jan 22. Biochim Biophys Acta. 2009. PMID: 19416656 Review.
Cited by
-
Sphingosine-1-phosphate metabolism: A structural perspective.Crit Rev Biochem Mol Biol. 2015;50(4):298-313. doi: 10.3109/10409238.2015.1039115. Epub 2015 Apr 29. Crit Rev Biochem Mol Biol. 2015. PMID: 25923252 Free PMC article. Review.
-
Structural and functional evidence that lipoprotein LpqN supports cell envelope biogenesis in Mycobacterium tuberculosis.J Biol Chem. 2019 Oct 25;294(43):15711-15723. doi: 10.1074/jbc.RA119.008781. Epub 2019 Aug 30. J Biol Chem. 2019. PMID: 31471317 Free PMC article.
-
Neuronal activity induces glucosylceramide that is secreted via exosomes for lysosomal degradation in glia.Sci Adv. 2022 Jul 15;8(28):eabn3326. doi: 10.1126/sciadv.abn3326. Epub 2022 Jul 13. Sci Adv. 2022. PMID: 35857503 Free PMC article.
-
Anti-inflammatory role of GM1 and other gangliosides on microglia.J Neuroinflammation. 2022 Jan 6;19(1):9. doi: 10.1186/s12974-021-02374-x. J Neuroinflammation. 2022. PMID: 34991625 Free PMC article.
-
The NMR-based characterization of the FTY720-SET complex reveals an alternative mechanism for the attenuation of the inhibitory SET-PP2A interaction.FASEB J. 2019 Jun;33(6):7647-7666. doi: 10.1096/fj.201802264R. Epub 2019 Mar 27. FASEB J. 2019. PMID: 30917007 Free PMC article.
References
-
- Hanada K, Kumagai K, Tomishige N, Kawano M. Biochim Biophys Acta. 2007;1771:644–653. - PubMed
-
- Hanada K, Hara T, Fukasawa M, Yamaji A, Umeda M, Nishijima M. J Biol Chem. 1998;273:33787–33794. - PubMed
-
- Funakoshi T, Yasuda S, Fukasawa M, Nishijima M, Hanada K. J Biol Chem. 2000;275:29938–29945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

