Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions
- PMID: 18184808
- PMCID: PMC2206616
- DOI: 10.1073/pnas.0708947105
Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions
Abstract
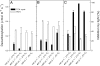
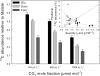
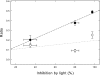
Day respiration is the process by which nonphotorespiratory CO2 is produced by illuminated leaves. The biological function of day respiratory metabolism is a major conundrum of plant photosynthesis research: because the rate of CO2 evolution is partly inhibited in the light, it is viewed as either detrimental to plant carbon balance or necessary for photosynthesis operation (e.g., in providing cytoplasmic ATP for sucrose synthesis). Systematic variations in the rate of day respiration under contrasting environmental conditions have been used to elucidate the metabolic rationale of respiration in the light. Using isotopic techniques, we show that both glycolysis and the tricarboxylic acid cycle activities are inversely related to the ambient CO2/O2 ratio: day respiratory metabolism is enhanced under high photorespiratory (low CO2) conditions. Such a relationship also correlates with the dihydroxyacetone phosphate/Glc-6-P ratio, suggesting that photosynthetic products exert a control on day respiration. Thus, day respiration is normally inhibited by phosphoryl (ATP/ADP) and reductive (NADH/NAD) poise but is up-regulated by photorespiration. Such an effect may be related to the need for NH2 transfers during the recovery of photorespiratory cycle intermediates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Carbon allocation to major metabolites in illuminated leaves is not just proportional to photosynthesis when gaseous conditions (CO2 and O2 ) vary.New Phytol. 2018 Apr;218(1):94-106. doi: 10.1111/nph.14984. Epub 2018 Jan 18. New Phytol. 2018. PMID: 29344970
-
Leaf day respiration involves multiple carbon sources and depends on previous dark metabolism.Plant Cell Environ. 2024 Jun;47(6):2146-2162. doi: 10.1111/pce.14871. Epub 2024 Mar 5. Plant Cell Environ. 2024. PMID: 38444114
-
12CO2 emission from different metabolic pathways measured in illuminated and darkened C3 and C4 leaves at low, atmospheric and elevated CO2 concentration.J Exp Bot. 2003 Jul;54(388):1761-9. doi: 10.1093/jxb/erg187. Epub 2003 May 28. J Exp Bot. 2003. PMID: 12773522
-
Current methods for estimating the rate of photorespiration in leaves.Plant Biol (Stuttg). 2013 Jul;15(4):648-55. doi: 10.1111/j.1438-8677.2012.00694.x. Epub 2012 Nov 27. Plant Biol (Stuttg). 2013. PMID: 23186383 Review.
-
Net photosynthetic CO2 assimilation: more than just CO2 and O2 reduction cycles.New Phytol. 2019 Jul;223(2):520-529. doi: 10.1111/nph.15828. Epub 2019 Apr 12. New Phytol. 2019. PMID: 30927445 Review.
Cited by
-
Soybean Inoculated With One Bradyrhizobium Strain Isolated at Elevated [CO2] Show an Impaired C and N Metabolism When Grown at Ambient [CO2].Front Plant Sci. 2021 May 20;12:656961. doi: 10.3389/fpls.2021.656961. eCollection 2021. Front Plant Sci. 2021. PMID: 34093614 Free PMC article.
-
Differential physiological responses to environmental change promote woody shrub expansion.Ecol Evol. 2013 May;3(5):1149-62. doi: 10.1002/ece3.525. Epub 2013 Mar 13. Ecol Evol. 2013. PMID: 23762503 Free PMC article.
-
Diurnal changes in mitochondrial function reveal daily optimization of light and dark respiratory metabolism in Arabidopsis.Mol Cell Proteomics. 2010 Oct;9(10):2125-39. doi: 10.1074/mcp.M110.001214. Epub 2010 Jul 2. Mol Cell Proteomics. 2010. PMID: 20601493 Free PMC article.
-
Targeted quantitative profiling of metabolites and gene transcripts associated with 4-aminobutyrate (GABA) in apple fruit stored under multiple abiotic stresses.Hortic Res. 2018 Dec 1;5:61. doi: 10.1038/s41438-018-0069-3. eCollection 2018. Hortic Res. 2018. PMID: 30510768 Free PMC article.
-
Leaf-level metabolic changes in response to drought affect daytime CO2 emission and isoprenoid synthesis pathways.Tree Physiol. 2023 Nov 13;43(11):1917-1932. doi: 10.1093/treephys/tpad094. Tree Physiol. 2023. PMID: 37552065 Free PMC article.
References
-
- Krebs HA, Johnson WA. Enzymologia. 1937;4:148–156.
-
- Nunes-Nesi A, Sweetlove LJ, Fernie AR. Physiol Plant. 2007;129:45–56.
-
- Atkin OK, Millar AH, Gardeström P, Day DA. In: Photosynthesis, Physiology, and Metabolism. Leegood RC, Sharkey TD, von Caemmerer S, editors. London: Kluwer; 2000. pp. 203–220.
-
- Cornic G. Physiol Vég. 1973;11:663–679.

