Considerable differences in vaccine immunogenicities and efficacies related to the diluent used for aluminum hydroxide adjuvant
- PMID: 18184821
- PMCID: PMC2268268
- DOI: 10.1128/CVI.00427-07
Considerable differences in vaccine immunogenicities and efficacies related to the diluent used for aluminum hydroxide adjuvant
Abstract
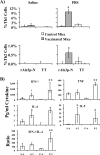
We are developing an anticandidal vaccine using the recombinant N terminus of Als3p (rAls3p-N). We report that although more rAls3p-N was bound by aluminum hydroxide diluted in saline than by aluminum hydroxide diluted in phosphate-buffered saline (PBS), its immunogenicity and efficacy were superior in PBS. Thus, protein binding, by itself, may not predict the efficacy of some vaccines with aluminum adjuvants.
Figures

References
-
- Baylor, N. W., W. Egan, and P. Richman. 2002. Aluminum salts in vaccines—US perspective. Vaccine 20(Suppl. 3):S18-S23. - PubMed
-
- Berthold, I., M. L. Pombo, L. Wagner, and J. L. Arciniega. 2005. Immunogenicity in mice of anthrax recombinant protective antigen in the presence of aluminum adjuvants. Vaccine 23:1993-1999. - PubMed
-
- Chang, M., Y. Shi, S. L. Nail, H. HogenEsch, S. B. Adams, J. L. White, and S. L. Hem. 2001. Degree of antigen adsorption in the vaccine or interstitial fluid and its effect on the antibody response in rabbits. Vaccine 19:2884-2889. - PubMed
-
- Flebbe, L. M., and H. Braley-Mullen. 1986. Immunopotentiating effects of the adjuvants SGP and Quil A. I. Antibody responses to T-dependent and T-independent antigens. Cell. Immunol. 99:119-127. - PubMed
-
- Gupta, R. K., E. H. Relyveld, E. B. Lindblad, B. Bizzini, S. Ben-Efraim, and C. K. Gupta. 1993. Adjuvants—a balance between toxicity and adjuvanticity. Vaccine 11:293-306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

