Vitrification of Carotid Artery Segments: An Integrated Study of Thermophysical Events and Functional Recovery Toward Scale-Up for Clinical Applications
- PMID: 18185850
- PMCID: PMC2180387
- DOI: 10.1089/cpt.2006.9994
Vitrification of Carotid Artery Segments: An Integrated Study of Thermophysical Events and Functional Recovery Toward Scale-Up for Clinical Applications
Abstract
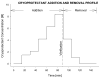
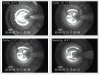
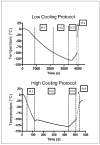
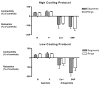
In recent years, ice-free cryopreservation by vitrification has been demonstrated to provide superior preservation of tissues compared with conventional freezing methods. To date, this has been accomplished almost exclusively for small model systems, whereas cryopreservation of large tissue samples-of a clinically useful size-continues to be hampered by thermomechanical effects that compromise the structure and function of the tissue. Reduction of mechanical stress is an integral condition of successful cryopreservation of large specimens. The current study focuses on the impact of sample size on both the physical events, observed by cryomacroscopy, and on the outcome on tissue function. To this end, the current study sought to address the question of functional recovery of vitrified carotid artery segments, processed as either artery rings (3-4 mm long) or segments (25 mm long) as selected models; the latter model represents a significant increase in sample size for evaluating the effects of vitrification. Tissue vitrification using an 8.4 M cryoprotectant cocktail solution (VS55) was achieved in 1-ml samples by imposing either a high (50-70 °C/min) or a low (2-3 °C/min) cooling rate, between -40°C and -100°C, and a high rewarming rate between -100°C and -40°C. Following cryoprotectant removal, the artery segments were cut into 3 to 4-mm rings for function testing on a contractility apparatus by measuring isometric responses to four agonist and antagonists (norepinephrine, phenylepinephrine, calcium ionophore, and sodium nitroprusside). In addition, nonspecific metabolic function of the vessel rings was determined using the REDOX indicator alamarBlue. Contractile function in response to the agonists norepinephrine and phenylepinephrine was maintained at the same level (350%) for the segments as for the rings, when compared with noncryopreserved control samples. Relaxation in response to the antagonists calcium ionophore and sodium nitroprusside was maintained at between 75% and 100% of control levels, irrespective of cooling rate or sample size. No evidence of macroscopic crystallization or fractures was observed by cryomacroscopy at the above rates in any of the samples. In conclusion, this study verifies that the rate of cooling and warming can be reduced from our baseline vitrification technique such that the function of larger tissue samples is not significantly different from that of smaller blood vessel rings. This represents a step toward the goal of achieving vitreous cryopreservation of large tissue samples without the destructive effect of thermal stresses.
Figures

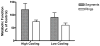
Similar articles
-
Cryopreservation of carotid artery segments via vitrification subject to marginal thermal conditions: correlation of freezing visualization with functional recovery.Cryobiology. 2008 Aug;57(1):1-8. doi: 10.1016/j.cryobiol.2008.03.002. Epub 2008 Mar 28. Cryobiology. 2008. PMID: 18490009 Free PMC article.
-
Vitreous tissue cryopreservation using a blood vessel model and cryomacroscopy for scale-up studies: Observations and mathematical modeling.Cryobiology. 2024 Dec;117:104976. doi: 10.1016/j.cryobiol.2024.104976. Epub 2024 Nov 7. Cryobiology. 2024. PMID: 39362358
-
A guide to successful mL to L scale vitrification and rewarming.Cryo Letters. 2022 Nov-Dec;43(6):316-321. Cryo Letters. 2022. PMID: 36629824 Free PMC article. Review.
-
Survival of mouse oocytes after being cooled in a vitrification solution to -196°C at 95° to 70,000°C/min and warmed at 610° to 118,000°C/min: A new paradigm for cryopreservation by vitrification.Cryobiology. 2011 Feb;62(1):1-7. doi: 10.1016/j.cryobiol.2010.10.159. Epub 2010 Nov 3. Cryobiology. 2011. PMID: 21055397 Free PMC article.
-
Cryopreservation: Vitrification and Controlled Rate Cooling.Methods Mol Biol. 2017;1590:41-77. doi: 10.1007/978-1-4939-6921-0_5. Methods Mol Biol. 2017. PMID: 28353262 Review.
Cited by
-
Polarized light scanning cryomacroscopy, part I: Experimental apparatus and observations of vitrification, crystallization, and photoelasticity effects.Cryobiology. 2016 Oct;73(2):261-71. doi: 10.1016/j.cryobiol.2016.06.005. Epub 2016 Jun 21. Cryobiology. 2016. PMID: 27343138 Free PMC article.
-
Phosphonate coating of commercial iron oxide nanoparticles for nanowarming cryopreserved samples.J Mater Chem B. 2022 May 18;10(19):3734-3746. doi: 10.1039/d1tb02483c. J Mater Chem B. 2022. PMID: 35466332 Free PMC article.
-
Role of cells in freezing-induced cell-fluid-matrix interactions within engineered tissues.J Biomech Eng. 2013 Sep;135(9):91001. doi: 10.1115/1.4024571. J Biomech Eng. 2013. PMID: 23719856 Free PMC article.
-
Rapid joule heating improves vitrification based cryopreservation.Nat Commun. 2022 Oct 12;13(1):6017. doi: 10.1038/s41467-022-33546-9. Nat Commun. 2022. PMID: 36224179 Free PMC article.
-
Thermostability of biological systems: fundamentals, challenges, and quantification.Open Biomed Eng J. 2011;5:47-73. doi: 10.2174/1874120701105010047. Epub 2011 Apr 12. Open Biomed Eng J. 2011. PMID: 21769301 Free PMC article.
References
-
- Song YC, Khirabadi BS, Lightfoot FG, Brockbank KGM, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nature Biotechnol. 2000;18:296–299. - PubMed
-
- Song YC, Lightfoot FG, Chen Z, Taylor MJ, Brockbank KGM. Vitreous Preservation of Rabbit Articular Cartilage. Cell Preserv Technol. 2004;2:67–74.
-
- Song YC, An YH, Kang QK, Li C, Boggs JM, Chen Z, Taylor MJ, Brockbank KGM. Vitreous preservation of articular cartilage grafts. J Invest Surg. 2004;17:1–6. - PubMed
-
- Taylor MJ, Song YC, Brockbank KGM. Vitrification in tissue preservation: new developments. In: Benson E, Fuller BJ, Lane N, editors. Life in the Frozen State. London: CRC Press; 2004. pp. 603–641.
-
- Rabin Y, Steif PS. Solid mechanics aspects of cryobiology. In: Baust JG, Baust JM, editors. Advances in Bio-preservation. Boca Raton: CRC Press; 2006. pp. 353–375.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
