Carbohydrate-mediated targeting of antigen to dendritic cells leads to enhanced presentation of antigen to T cells
- PMID: 18186095
- PMCID: PMC2700842
- DOI: 10.1002/cbic.200700310
Carbohydrate-mediated targeting of antigen to dendritic cells leads to enhanced presentation of antigen to T cells
Abstract
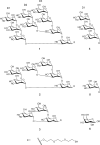
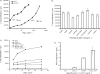
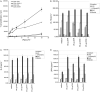
The unique therapeutic value of dendritic cells (DCs) for the treatment of allergy, autoimmunity and transplant rejection is predicated upon our ability to selectively deliver antigens, drugs or nucleic acids to DCs in vivo. Here we describe a method for delivering whole protein antigens to DCs based on carbohydrate-mediated targeting of DC-expressed lectins. A series of synthetic carbohydrates was chemically-coupled to a model antigen, ovalbumin (OVA), and each conjugate was evaluated for its ability to increase the efficiency of antigen presentation by murine DCs to OVA-specific T cells (CD4(+) and CD8(+)). In vitro data are presented that demonstrate that carbohydrate modification of OVA leads to a 50-fold enhancement of presentation of antigenic peptide to CD4(+) T cells. A tenfold enhancement is observed for CD8(+) T cells; this indicates that the targeted lectin(s) can mediate cross-presentation of antigens on MHC class I. Our data indicate that the observed enhancements in antigen presentation are unique to OVA that is conjugated to complex oligosaccharides, such as a high-mannose nonasaccharide, but not to monosaccharides. Taken together, our data suggest that a DC targeting strategy that is based upon carbohydrate-lectin interactions is a promising approach for enhancing antigen presentation via class I and class II molecules.
Figures


Similar articles
-
Targeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentation.Mol Immunol. 2009 Dec;47(2-3):164-74. doi: 10.1016/j.molimm.2009.09.026. Epub 2009 Oct 8. Mol Immunol. 2009. PMID: 19818504
-
Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance.J Exp Med. 2002 Dec 16;196(12):1627-38. doi: 10.1084/jem.20021598. J Exp Med. 2002. PMID: 12486105 Free PMC article.
-
Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells.J Immunol. 2001 May 1;166(9):5327-30. doi: 10.4049/jimmunol.166.9.5327. J Immunol. 2001. PMID: 11313367
-
Bacterial antigen delivery systems: phagocytic processing of bacterial antigens for MHC-I and MHC-II presentation to T cells.Behring Inst Mitt. 1997 Feb;(98):197-211. Behring Inst Mitt. 1997. PMID: 9382741 Review.
-
The interdisciplinary science of T-cell recognition.Adv Immunol. 2013;119:1-50. doi: 10.1016/B978-0-12-407707-2.00001-1. Adv Immunol. 2013. PMID: 23886063 Review.
Cited by
-
Immunogenicity Risk Assessment for an Engineered Human Cytokine Analogue Expressed in Different Cell Substrates.AAPS J. 2020 Apr 14;22(3):65. doi: 10.1208/s12248-020-00443-2. AAPS J. 2020. PMID: 32291556 Review.
-
Nanoparticles Presenting Potent TLR7/8 Agonists Enhance Anti-PD-L1 Immunotherapy in Cancer Treatment.Biomacromolecules. 2020 Sep 14;21(9):3704-3712. doi: 10.1021/acs.biomac.0c00812. Epub 2020 Aug 20. Biomacromolecules. 2020. PMID: 32816460 Free PMC article.
-
Specific Protein Antigen Delivery to Human Langerhans Cells in Intact Skin.Front Immunol. 2021 Oct 21;12:732298. doi: 10.3389/fimmu.2021.732298. eCollection 2021. Front Immunol. 2021. PMID: 34745102 Free PMC article.
-
Liposomes and nanotechnology in drug development: focus on oncotargets.Int J Nanomedicine. 2012;7:4943-51. doi: 10.2147/IJN.S30726. Epub 2012 Sep 14. Int J Nanomedicine. 2012. PMID: 23028222 Free PMC article.
-
Rational design, preparation and characterization of recombinant Ag85B variants and their glycoconjugates with T-cell antigenic activity against Mycobacterium tuberculosis.RSC Adv. 2018 Jun 26;8(41):23171-23180. doi: 10.1039/c8ra03535k. eCollection 2018 Jun 21. RSC Adv. 2018. PMID: 35540174 Free PMC article.
References
-
- Steinman RM, Hawiger D, Nussenzweig MC. Annu. Rev. Immunol. 2003;21:685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

