Adhesions in a murine flexor tendon graft model: autograft versus allograft reconstruction
- PMID: 18186128
- PMCID: PMC2709286
- DOI: 10.1002/jor.20531
Adhesions in a murine flexor tendon graft model: autograft versus allograft reconstruction
Abstract
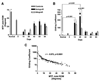
Reconstruction of flexor tendons often results in adhesions that compromise joint flexion. Little is known about the factors involved in the formation of flexor tendon graft adhesions. In this study, we developed and characterized a novel mouse model of flexor digitorum longus (FDL) tendon reconstruction with live autografts or reconstituted freeze-dried allografts. Grafted tendons were evaluated at multiple time points up to 84 days post-reconstruction. To assess the flexion range of the metatarsophalangeal joint, we developed a quantitative outcome measure proportional to the resistance to tendon gliding due to adhesions, which we termed the Gliding Coefficient. At 14 days post-grafting, the Gliding Coefficient was 29- and 26-fold greater than normal FDL tendon for both autografts and allografts, respectively (p < 0.001), and subsequently doubled for 28-day autografts. Interestingly, there were no significant differences in maximum tensile force or stiffness between live autograft and freeze-dried allograft repairs over time. Histologically, autograft healing was characterized by extensive remodeling and exuberant scarring around both the ends and the body of the graft, whereas allograft scarring was abundant only near the graft-host junctions. Gene expression of GDF-5 and VEGF were significantly increased in 28-day autografts compared to allografts and to normal tendons. These results suggest that the biomechanical advantages for tendon reconstruction using live autografts over devitalized allografts are minimal. This mouse model can be useful in elucidating the molecular mechanisms in tendon repair and can aid in preliminary screening of molecular treatments of flexor tendon adhesions.
(c) 2008 Orthopaedic Research Society.
Figures






References
-
- Taras JS, Lamb MJ. Treatment of flexor tendon injuries: surgeons' perspective. Journal of Hand Therapy. 1999;12:141–148. - PubMed
-
- Bunnell S. The injured hand; principles of treatment. Ind Med Surg. 1953;22:251–254. - PubMed
-
- Lilly SI, Messer TM. Complications after treatment of flexor tendon injuries. J Am Acad Orthop Surg. 2006;14:387–396. - PubMed
-
- Stark HH, Anderson DR, Zemel NP, Boyes JH, Ashworth CR, Rickard TA. Bridge flexor tendon grafts. Clin Orthop Relat Res. 1989:51–59. - PubMed
-
- Taras JS, Kaufmann RA. Flexor Tendon Reconstruction. In: Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, editors. Green's Operative Hand Surgery. Fifth ed. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 241–276.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

