The role of Drosophila Merlin in spermatogenesis
- PMID: 18186933
- PMCID: PMC2253521
- DOI: 10.1186/1471-2121-9-1
The role of Drosophila Merlin in spermatogenesis
Abstract
Background: Drosophila Merlin, the homolog of the human Neurofibromatosis 2 (NF2) gene, is important for the regulation of cell proliferation and receptor endocytosis. Male flies carrying a Mer3 allele, a missense mutation (Met177-->Ile) in the Merlin gene, are viable but sterile; however, the cause of sterility is unknown.
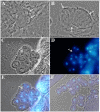
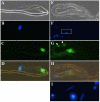
Results: Testis examination reveals that hemizygous Mer3 mutant males have small seminal vesicles that contain only a few immotile sperm. By cytological and electron microscopy analyses of the Mer3, Mer4 (Gln170-->stop), and control testes at various stages of spermatogenesis, we show that Merlin mutations affect meiotic cytokinesis of spermatocytes, cyst polarization and nuclear shaping during spermatid elongation, and spermatid individualization. We also demonstrate that the lethality and sterility phenotype of the Mer4 mutant is rescued by the introduction of a wild-type Merlin gene. Immunostaining demonstrates that the Merlin protein is redistributed to the area associated with the microtubules of the central spindle in telophase and its staining is less in the region of the contractile ring during meiotic cytokinesis. At the onion stage, Merlin is concentrated in the Nebenkern of spermatids, and this mitochondrial localization is maintained throughout sperm formation. Also, Merlin exhibits punctate staining in the acrosomal region of mature sperm.
Conclusion: Merlin mutations affect spermatogenesis at multiple stages. The Merlin protein is dynamically redistributed during meiosis of spermatocytes and is concentrated in the Nebenkern of spermatids. Our results demonstrated for the first time the mitochondrial localization of Merlin and suggest that Merlin may play a role in mitochondria formation and function during spermatogenesis.
Figures




References
-
- Lindsley DL, Tokuyasu KT. Spermatogenesis. In: Ashburner M, Wright TR, editor. Genetics and Biology of Drosophila. 2. Academic Press, NY, NY; 1980. pp. 225–294.
-
- Fuller M. Spermatogenesis. In: Bate M, Arias AM, editor. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, NY; 1993. pp. 71–147.
-
- Cross DP, Shellenbargerr DL. The dynamics of Drosophila melanogaster spermatogenesis in in vitro cultures. J Embryol Exp Morph. 1979;53:345–351. - PubMed
-
- Fabrizio J, Hime G, Lemmon S, Bazinet C. Genetic dissection of sperm individualization in Drosophila melanogaster. Development. 1998;125:1833–1843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

