Poor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help?
- PMID: 18186995
- PMCID: PMC2148237
- DOI: 10.3399/bjgp08X263802
Poor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help?
Abstract
Background: Poor adherence with inhaled corticosteroids is an important problem in asthma management. Previous approaches to improving adherence have had limited success.
Aim: To determine whether treatment with a single inhaler containing a long-acting beta(2)-agonist and a corticosteroid for maintenance treatment and symptom relief can overcome the problem of poor adherence with inhaled corticosteroids.
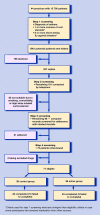
Design of study: Randomised, parallel group, open-label trial.
Setting: Forty-four general practices in Nottinghamshire.
Method: Participants who used less than 70% of their prescribed dose of inhaled corticosteroid and had poorly controlled asthma were randomised to budesonide 200 microg one puff twice daily plus their own short-acting beta(2)-agonist as required (control group), or budesonide/formoterol 200/6 microg one puff once daily and as required (active group) for 6 months. The primary outcome was inhaled corticosteroid dose.
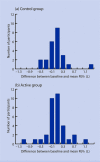
Results: Seventy-one participants (35 control, 36 active group) were randomised. Adherence with budesonide in the control group was approximately 60% of the prescribed dose. Participants in the active group used approximately 80% more budesonide than participants in the control group (448 versus 252 microg/day, mean difference 196 mug, 95% confidence interval 113 to 279; P<0.001) and were less likely to withdraw from the study (3 versus 13; P<0.01). No safety issues were identified.
Conclusion: Using a single inhaler for both maintenance treatment and symptom relief approximately doubled the dose of inhaled corticosteroid taken, suggesting this could be a useful strategy to overcome the problems related to poor adherence with inhaled corticosteroids.
Figures

References
-
- Senthilselvan A, Lawson JA, Rennie DC, Dosman JA. Regular use of corticosteroids and low use of short-acting beta2-agonists can reduce asthma hospitalisation. Chest. 2005;127(4):242–251. - PubMed
-
- Krishnan JA, Riekert KA, McCoy JV, et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170(12):1281–1285. - PubMed
-
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288–1293. - PubMed
-
- Cerveri I, Locatelli F, Zoia MC, et al. International variations in asthma treatment compliance: the results of the European Community Respiratory Health Survey (ECRHS) Eur Respir J. 1999;14(2):288–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
