Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites
- PMID: 18187451
- PMCID: PMC3164837
- DOI: 10.1242/jcs.012666
Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites
Erratum in
- J Cell Sci. 2008 Feb 1;121(Pt 3):405. Ohi, Ryoma [added]
Abstract
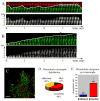
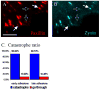
An organized microtubule array is essential for the polarized motility of fibroblasts. Dynamic microtubules closely interact with focal adhesion sites in migrating cells. Here, we examined the effect of focal adhesions on microtubule dynamics. We observed that the probability of microtubule catastrophes (transitions from growth to shrinkage) was seven times higher at focal adhesions than elsewhere. Analysis of the dependence between the microtubule growth rate and catastrophe probability throughout the cytoplasm revealed that a nonspecific (mechanical or spatial) factor provided a minor contribution to the catastrophe induction by decreasing microtubule growth rate at adhesions. Strikingly, at the same growth rate, the probability of catastrophes was significantly higher at adhesions than elsewhere, indicative of a site-specific biochemical trigger. The observed catastrophe induction occurred at adhesion domains containing the scaffolding protein paxillin that has been shown previously to interact with tubulin. Furthermore, replacement of full-length paxillin at adhesion sites by microinjected paxillin LIM2-LIM3 domains suppressed microtubule catastrophes exclusively at adhesions. We suggest that paxillin influences microtubule dynamics at focal adhesions by serving as a scaffold for a putative catastrophe factor and/or regulating its exposure to microtubules.
Figures





References
-
- Amos LA, Schlieper D. Microtubules and maps. Adv Protein Chem. 2005;71:257–98. - PubMed
-
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–68. - PubMed
-
- Axelrod D. Total internal reflection fluorescence microscopy. Methods Cell Biol. 1989;30 :245–70. - PubMed
-
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

