'Pressure-flow'-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria
- PMID: 18187469
- PMCID: PMC2375664
- DOI: 10.1113/jphysiol.2007.149294
'Pressure-flow'-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria
Abstract
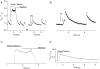
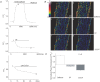
Cardiac myocytes, in the intact heart, are exposed to shear/fluid forces during each cardiac cycle. Here we describe a novel Ca(2+) signalling pathway, generated by 'pressurized flows' (PFs) of solutions, resulting in the activation of slowly developing ( approximately 300 ms) Ca(2+) transients lasting approximately 1700 ms at room temperature. Though subsequent PFs (applied some 10-30 s later) produced much smaller or undetectable responses, such transients could be reactivated following caffeine- or KCl-induced Ca(2+) releases, suggesting that a small, but replenishable, Ca(2+) pool serves as the source for their activation. PF-triggered Ca(2+) transients could be activated in Ca(2+)-free solutions or in solutions that block voltage-gated Ca(2+) channels, stretch-activated channels (SACs), or the Na(+)-Ca(2+) exchanger (NCX), using Cd(2+), Gd(3+), or Ni(2+), respectively. PF-triggered Ca(2+) transients were significantly smaller in quiescent than in electrically paced myocytes. Paced Ca(2+) transients activated at the peak of PF-triggered Ca(2+) transients were not significantly smaller than those produced normally, suggesting functionally separate Ca(2+) pools for paced and PF-triggered transients. Suppression of nitric oxide (NO) or IP(3) signalling pathways did not alter the PF-triggered Ca(2+) transients. On the other hand, mitochondrial metabolic uncoupler FCCP, in the presence of oligomycin (to prevent ATP depletion), reversibly suppressed PF-triggered Ca(2+) transients, as did the mitochondrial Ca(2+) uniporter (mCU) blocker, Ru360. Reducing agent DTT and reactive oxygen species (ROS) scavenger tempol, as well as mitochondrial NCX (mNCX) blocker CGP-37157, inhibited PF-triggered Ca(2+) transients. In rhod-2 AM-loaded and permeabilized cells, confocal imaging of mitochondrial Ca(2+) showed a transient increase in Ca(2+) on caffeine exposure and a decrease in mitochondrial Ca(2+) on application of PF pulses of solution. These signals were strongly suppressed by either Na(+)-free or CGP-37157-containing solutions, implicating mNCX in mediating the Ca(2+) release process. We conclude that subjecting rat cardiac myocytes to pressurized flow pulses of solutions triggers the release of Ca(2+) from a store that appears to access mitochondrial Ca(2+).
Figures
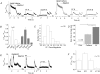
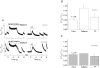



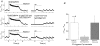
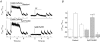



References
-
- Ali MH, Pearlstein DP, Mathieu CE, Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L486–L496. - PubMed
-
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. - PubMed
-
- Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–21488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

