Block of inhibitory junction potentials and TREK-1 channels in murine colon by Ca2+ store-active drugs
- PMID: 18187470
- PMCID: PMC2375637
- DOI: 10.1113/jphysiol.2007.148718
Block of inhibitory junction potentials and TREK-1 channels in murine colon by Ca2+ store-active drugs
Abstract
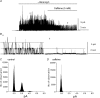
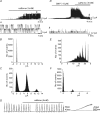
Post-junctional enteric inhibitory responses are composed of at least two components attributed to the release of a purine and nitric oxide (NO). The nitrergic component is characterized by membrane potential hyperpolarization; however, the conductances involved and the role of Ca(2+) stores in regulating these conductances are controversial. Conventional microelectrode recordings were performed in intact muscle strips and whole-cell voltage clamp experiments were performed on freshly dispersed cells and COS7 cells stably transfected with TREK-1 channels. Here we show that several Ca(2+) store-active compounds, including caffeine, ryanodine, and cyclopiazonic acid, reduce inhibitory junction potentials and responses to sodium nitroprusside in murine colonic muscles. We previously proposed that two-pore K(+) channels of the TREK family mediate a portion of the hyperpolarization response to NO in colonic muscles. We tested the effects of Ca(2+) store-active drugs in COS cells expressing murine TREK-1 channels and found these compounds block TREK-1 currents. These effects were greatly attenuated by dialysing cells with protein kinase A inhibitory peptide (PKAI). Caffeine also blocked stretch-dependent K(+) (SDK) channels, thought to be due to expression of TREK channels, in colonic myocytes, but these effects were not apparent in excised patches. Taken together our data show that Ca(2+) store-active compounds inhibit TREK-1 channels, native SDK channels, and nitrergic inhibitory junction potentials. These effects appear to be due, in part, to the cAMP/PKA stimulatory actions of these drugs and inhibitory effects of TREK channels.
Figures






Similar articles
-
FK506-binding protein (FKBP12) regulates ryanodine receptor-evoked Ca2+ release in colonic but not aortic smooth muscle.Cell Calcium. 2008 Jun;43(6):539-49. doi: 10.1016/j.ceca.2007.09.002. Epub 2007 Oct 24. Cell Calcium. 2008. PMID: 17950843
-
TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission.J Biol Chem. 2001 Nov 23;276(47):44338-46. doi: 10.1074/jbc.M108125200. Epub 2001 Sep 17. J Biol Chem. 2001. PMID: 11560940
-
TREK-1 channels do not mediate nitrergic neurotransmission in circular smooth muscle from the lower oesophageal sphincter.Br J Pharmacol. 2010 Jan 1;159(2):362-73. doi: 10.1111/j.1476-5381.2009.00531.x. Epub 2009 Dec 4. Br J Pharmacol. 2010. PMID: 20002101 Free PMC article.
-
Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation.J Physiol. 2006 Jan 1;570(Pt 1):37-43. doi: 10.1113/jphysiol.2005.098897. Epub 2005 Oct 20. J Physiol. 2006. PMID: 16239268 Free PMC article. Review.
-
Store-operated calcium entry and intracellular calcium release channels in airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2004 May;286(5):L907-8. doi: 10.1152/ajplung.00410.2003. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 15064237 Review. No abstract available.
Cited by
-
Expression of stretch-activated two-pore potassium channels in human myometrium in pregnancy and labor.PLoS One. 2010 Aug 25;5(8):e12372. doi: 10.1371/journal.pone.0012372. PLoS One. 2010. PMID: 20811500 Free PMC article.
-
Negative Influence by the Force: Mechanically Induced Hyperpolarization via K2P Background Potassium Channels.Int J Mol Sci. 2021 Aug 23;22(16):9062. doi: 10.3390/ijms22169062. Int J Mol Sci. 2021. PMID: 34445768 Free PMC article. Review.
-
Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract.Br J Pharmacol. 2019 Jan;176(2):212-227. doi: 10.1111/bph.14459. Epub 2018 Sep 3. Br J Pharmacol. 2019. PMID: 30063800 Free PMC article. Review.
-
Mechanosensitive Piezo Channels in the Gastrointestinal Tract.Curr Top Membr. 2017;79:219-244. doi: 10.1016/bs.ctm.2016.11.003. Epub 2017 Jan 7. Curr Top Membr. 2017. PMID: 28728818 Free PMC article. Review.
-
Protein kinase A and C regulate leak potassium currents in freshly isolated vascular myocytes from the aorta.PLoS One. 2013 Sep 23;8(9):e75077. doi: 10.1371/journal.pone.0075077. eCollection 2013. PLoS One. 2013. PMID: 24086441 Free PMC article.
References
-
- Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol Cell Physiol. 2000;279:C126–C135. - PubMed
-
- Beavo JA, Rogers NL, Crofford OB, Hardman JG, Sutherland EW, Newman EV. Effects of xanthine derivatives on lipolysis and on adenosine 3′,5′-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970;6:597–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

