Trauma and hemorrhage-induced acute hepatic insulin resistance: dominant role of tumor necrosis factor-alpha
- PMID: 18187553
- PMCID: PMC2329283
- DOI: 10.1210/en.2007-0922
Trauma and hemorrhage-induced acute hepatic insulin resistance: dominant role of tumor necrosis factor-alpha
Abstract
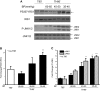
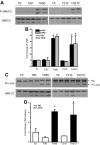
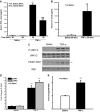
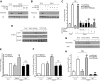
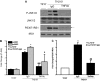
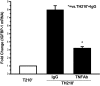
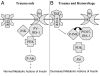
It has long been known that injury, infections, and other critical illnesses are often associated with hyperglycemia and hyperinsulinemia. Mortality of critically ill patients is greatly reduced by intensive insulin therapy, suggesting the significance of reversing or compensating for the development of acute insulin resistance. However, the development of acute injury/infection-induced insulin resistance is poorly studied, much less than the chronic diseases associated with insulin resistance, such as type 2 diabetes and obesity. We previously found that insulin resistance develops acutely in the liver after trauma and hemorrhage. The present study was designed to begin to understand the first steps in the development of trauma and hemorrhage-induced acute hepatic insulin resistance in an animal model of injury and blood loss similar to traumatic or surgical injury and hemorrhage. We present novel data that indicate that hepatic insulin resistance increased dramatically with an increasing extent of hemorrhage. With increasing extent of blood loss, there were increases in serum TNF-alpha levels, phosphorylation of liver insulin receptor substrate-1 on serine 307, and liver c-Jun N-terminal kinase activation/phosphorylation. Exogenous TNF-alpha infusion increased c-Jun N-terminal kinase phosphorylation and insulin receptor substrate-1 serine 307 phosphorylation, and inhibited insulin-induced signaling in liver. Conversely, neutralizing TNF-alpha antibody treatment reversed many of the hemorrhage-induced changes in hepatic insulin signaling. Our data indicate that the acute development of insulin resistance after trauma and hemorrhage may have some similarities to the insulin resistance that occurs in chronic diseases. However, because so little is known about this acute insulin-resistant state, much more needs to be done before we can attain a level of understanding similar to that of chronic states of insulin resistance.
Figures



References
-
- Del Aguila LF, Krishnan RK, Ulbrecht JS, Farrell PA, Correll PH, Lang, CH, Zierath JR, Kirwan JP 2000 Muscle damage impairs insulin stimulation of IRS-1, PI 3-kinase, and Akt-kinase in human skeletal muscle. Am J Physiol Endocrinol Metab 279:E206–E212 - PubMed
-
- Chaudry IH, Sayeed MM, Baue AE 1974 Insulin resistance in experimental shock. Arch Surg 109:412–415 - PubMed
-
- Ikezu T, Okamoto T, Yonezawa K, Tompkins RG, Martyn JA 1997 Analysis of thermal injury-induced insulin resistance in rodents. Implication of postreceptor mechanisms. J Biol Chem 272:25289–25295 - PubMed
-
- Carter EA 1998 Insulin resistance in burns and trauma. Nutr Rev 56(1 Pt 2):S170–S176 - PubMed
-
- Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R 2001 Intensive insulin therapy in the critically ill patients. N Engl J Med 345:1359–1367 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

