Lupus nephritis: the central role of nucleosomes revealed
- PMID: 18187568
- PMCID: PMC2312358
- DOI: 10.2353/ajpath.2008.070563
Lupus nephritis: the central role of nucleosomes revealed
Abstract
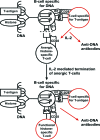
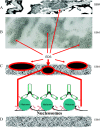
Systemic lupus erythematosus (SLE) is an autoimmune syndrome characterized by autoantibodies to nuclear constituents. Some of these antibodies are diagnostically important, whereas others act as disease-modifying factors. One clinically important factor is autoantibodies against dsDNA and nucleosomes, which have overlapping diagnostic and nephritogenic impact in SLE. Although a scientific focus for 5 decades, the molecular and cellular origin of these antibodies, and why they are associated with lupus nephritis, is still not fully understood. A consensus has, however, evolved that antibodies to dsDNA and nucleosomes are central pathogenic factors in the development of lupus nephritis. In contrast, no agreement has been reached as to which glomerular structures are bound by nephritogenic anti-nucleosome antibodies in vivo. Mutually contradictory paradigms and models have evolved simply because we still lack precise and conclusive data to provide definitive insight into how autoantibodies induce lupus nephritis and which specificity is critical in the nephritic process(es). In this review, data demonstrating the central role of nucleosomes in inducing and binding potentially nephritogenic antibodies to DNA and nucleosomes are presented and discussed. These autoimmune-inducing processes are discussed in the context of Matzinger's danger model (Matzinger P: Friendly and dangerous signals: is the tissue in control? Nat Immunol 2007, 8:11-13; Matzinger P: The danger model: a renewed sense of self. Science 2002, 296:301-305; Matzinger P: Tolerance, danger, and the extended family. Annu Rev Immunol 1994, 12:991-1045) and Medzhitov's and Janeway's (Medzhitov R, Janeway CA Jr: Decoding the patterns of self and nonself by the innate immune system. Science 2002, 296:298-300; Medzhitov R, Janeway CA Jr: How does the immune system distinguish self from nonself? Semin Immunol 2000, 12:185-188; Janeway CA Jr, Medzhitov R: Innate immune recognition. Annu Rev Immunol 2002, 20:197-216) distinction of noninfectious self (NIS) and infectious nonself (INS). The mechanisms leading to production of potentially nephritogenic anti-nucleosome antibodies and to overt lupus nephritis are interpreted in the context of these paradigms.
Figures

Similar articles
-
Autoimmunity against nucleosomes and lupus nephritis.Ann Med Interne (Paris). 1996;147(7):485-9. Ann Med Interne (Paris). 1996. PMID: 9092359 Review.
-
Critical comparative analyses of anti-alpha-actinin and glomerulus-bound antibodies in human and murine lupus nephritis.Arthritis Rheum. 2006 Mar;54(3):914-26. doi: 10.1002/art.21622. Arthritis Rheum. 2006. PMID: 16508974
-
Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes.J Am Soc Nephrol. 2009 Apr;20(4):696-704. doi: 10.1681/ASN.2008010112. J Am Soc Nephrol. 2009. PMID: 19329762 Review.
-
Lupus nephritis: a nucleosome waste disposal defect?J Nephrol. 2002 Nov-Dec;15 Suppl 6:S1-10. J Nephrol. 2002. PMID: 12515368 Review.
-
Anti-DNA antibody subpopulations and lupus nephritis.Autoimmun Rev. 2004 Feb;3(2):1-6. doi: 10.1016/S1568-9972(03)00081-8. Autoimmun Rev. 2004. PMID: 15003181 Review.
Cited by
-
Development of self-reactive germinal center B cells and plasma cells in autoimmune Fc gammaRIIB-deficient mice.J Exp Med. 2010 Nov 22;207(12):2767-78. doi: 10.1084/jem.20100171. Epub 2010 Nov 15. J Exp Med. 2010. PMID: 21078890 Free PMC article.
-
Molecular and Immunological Basis of Tubulo-Interstitial Injury in Lupus Nephritis: a Comprehensive Review.Clin Rev Allergy Immunol. 2017 Apr;52(2):149-163. doi: 10.1007/s12016-016-8533-z. Clin Rev Allergy Immunol. 2017. PMID: 26961386 Review.
-
Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice.PLoS One. 2009 Dec 29;4(12):e8474. doi: 10.1371/journal.pone.0008474. PLoS One. 2009. PMID: 20041189 Free PMC article.
-
Philosophical and distinct SLE epitomes: dogmas in conflict with evidences and an intellectual dissonance between established pathophysiological models.Front Immunol. 2025 Jul 24;16:1580664. doi: 10.3389/fimmu.2025.1580664. eCollection 2025. Front Immunol. 2025. PMID: 40777033 Free PMC article. Review.
-
Dangers within: DAMP responses to damage and cell death in kidney disease.J Am Soc Nephrol. 2011 Mar;22(3):416-25. doi: 10.1681/ASN.2010040430. Epub 2011 Feb 18. J Am Soc Nephrol. 2011. PMID: 21335516 Free PMC article. Review.
References
-
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. - PubMed
-
- Fismen S, Rekvig OP, Mortensen E. Pathogenesis of SLE dermatitis—a reflection of the process in SLE nephritis? Curr Rheum Rev. 2007;3:1–7.
-
- Rekvig OP, Kalaaji M, Nossent H. Anti-DNA antibody subpopulations and lupus nephritis. Autoimmun Rev. 2004;3:1–6. - PubMed
-
- Rekvig OP, Nossent JC. Anti-double-stranded DNA antibodies, nucleosomes, and systemic lupus erythematosus: a time for new paradigms? Arthritis Rheum. 2003;48:300–312. - PubMed
-
- Berden JH, Licht R, Van Bruggen MC, Tax WJ. Role of nucleosomes for induction and glomerular binding of autoantibodies in lupus nephritis. Curr Opin Nephrol Hypertens. 1999;8:299–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

