Role of Gas6 in erythropoiesis and anemia in mice
- PMID: 18188450
- PMCID: PMC2176185
- DOI: 10.1172/JCI30375
Role of Gas6 in erythropoiesis and anemia in mice
Abstract
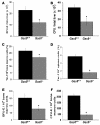
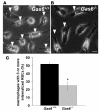
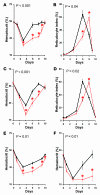
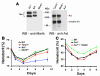
Many patients with anemia fail to respond to treatment with erythropoietin (Epo), a commonly used hormone that stimulates erythroid progenitor production and maturation by human BM or by murine spleen. The protein product of growth arrest-specific gene 6 (Gas6) is important for cell survival across several cell types, but its precise physiological role remains largely enigmatic. Here, we report that murine erythroblasts released Gas6 in response to Epo and that Gas6 enhanced Epo receptor signaling by activating the serine-threonine kinase Akt in these cells. In the absence of Gas6, erythroid progenitors and erythroblasts were hyporesponsive to the survival activity of Epo and failed to restore hematocrit levels in response to anemia. In addition, Gas6 may influence erythropoiesis via paracrine erythroblast-independent mechanisms involving macrophages. When mice with acute anemia were treated with Gas6, the protein normalized hematocrit levels without causing undesired erythrocytosis. In a transgenic mouse model of chronic anemia caused by insufficient Epo production, Gas6 synergized with Epo in restoring hematocrit levels. These findings may have implications for the treatment of patients with anemia who fail to adequately respond to Epo.
Figures



References
-
- Weatherall D.J., Provan A.B. Red cells I: inherited anaemias. Lancet. 2000;355:1169–1175. - PubMed
-
- Richmond T.D., Chohan M., Barber D.L. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. - PubMed
-
- Koury M.J., Bondurant M.C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–381. - PubMed
-
- Krystal G. A simple microassay for erythropoietin based on 3H-thymidine incorporation into spleen cells from phenylhydrazine treated mice. Exp. Hematol. 1983;11:649–660. - PubMed
-
- Blumenauer B., Cranney A., Clinch J., Tugwell P. Quality of life in patients with rheumatoid arthritis: which drugs might make a difference? Pharmacoeconomics. 2003;21:927–940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

